a. In vapor-liquid equilibrium in a binary mixture, both components are generally present in both phases. How
Question:
a. In vapor-liquid equilibrium in a binary mixture, both components are generally present in both phases. How many degrees of freedom are there for such a system?
b. The reaction between nitrogen and hydrogen to form ammonia occurs in the gas phase. How many degrees of freedom are there for this system?
c. Steam and coal react at high temperatures to form hydrogen, carbon monoxide, carbon dioxide, and methane. The following reactions have been suggested as being involved in the chemical transformation:
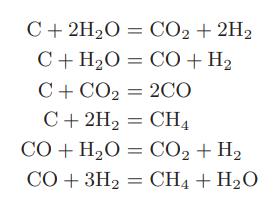
How many degrees of freedom are there for this system?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:





