A rough rule of thumb in polymer solution theory is that a 4 molar aqueous polymer solution
Question:
A rough rule of thumb in polymer solution theory is that a 4 molar aqueous polymer solution will have an osmotic pressure of approximately 100 bar. Is this rule of thumb in approximate agreement with Eq.11.5-5?
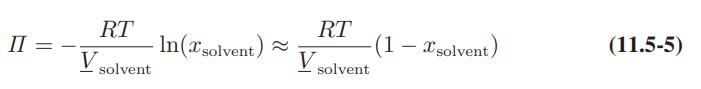
Transcribed Image Text:
II = RT V solvent -In(xsolvent) RT VSC solvent (1 - xsolvent) (11.5-5)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
Yes the rule of thumb that a 4 molar aqueous polymer solution will have an osmotic pressure of appro...View the full answer

Answered By

PU Student
cost accounting
financial accounting
auditing
internal control
business analyst
tax
i have 3 years experience in field of management & auditing in different multinational firms. i also have 16 months experience as an accountant in different international firms. secondary school certification.
higher secondary school certification.
bachelors in mathematics.
cost & management accountant
4.80+
4+ Reviews
10+ Question Solved
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:
Students also viewed these Engineering questions
-
A rough rule of thumb is that light from the Sun reaches the Earth in about 8 minutes. Use this estimate along with the known speed of light to get an approximate value for the distance from the...
-
Insulin is a protein important in the metabolism of sugar. Its molar mass can be determined by means of an osmotic pressure experiment. A 50.0-mg sample of insulin was dissolved in enough water to...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
Find the equation for the lower half of the circle x + (y-8) = 7. Put the equation in the form y = g(x), and then enter g(x) into the answer box below. Enter your answer as a symbolic function of x,...
-
On July 1, 2008, Baker Corporation sold equipment it had recently purchased to an unaffiliated company for $570,000. The equipment had a book value on Bakers books of $450,000 and a remaining life of...
-
Transmission through thin layers in Figure light is incident perpendicularly on a thin layer of material 2 that lies between (thicker) materials 1 and 3. (The rays are tilted only for clarity.) Part...
-
XCBob, Inc. is an online motorcycle and ATV parts business from the owners home. XCBob entered into oral agreements with the defendant, Ed Tucker Distributers, for purchases of various materials to...
-
The Windshield People repair chips in car windshields in the companys home county. Rocky Chip, the owner, incurred the following operating costs for the month of February 2012: Salaries and wages . ....
-
(20%) A Fabry-Perot resonant cavity consists of a thin glass plate that has a refractive index of n = 1.50 and a thickness of = 100 m. Its surfaces are coated such that its peak transmittance is 100%...
-
Joe Udel lives on the second floor of a house that is adjacent to a well of pure water, but city water comes out of his indoor plumbing. He would rather have pure well water. So he has developed the...
-
Derive a form of the Gibbs phase rule that applies to osmotic equilibrium.
-
If someone told you, policymakers couldnt vote themselves out of a phone booth, what type of policy lag is he or she referring to?
-
what are the principles of forecasting? Briefly explain different types of forecasting methods?
-
What are the different type of product classification Briefly explain consumer product classification
-
Explain how Peter Diamandis' 6D's of exponential growth and his 8 converging exponential technologies relate to healthcare innovation. How would there be entrepreneurial opportunity for growth...
-
The Conditions of Tendering: Explain for better understanding the 5 main contents below and the reasons for requiring these conditions. Eligibility of tenderers Validity period of tenderers...
-
Three authors who explain the importance of ERP CRM Cybersecurity and MRP in Global Logistics Distribution and Business Intelligence Quotes with authors about what they say about these topics....
-
How are realized and unrealized investment gains reported in investment trust funds?
-
The following T-accounts show postings of selected transactions. Indicate the journal used in recording each of these postings a through e. Cash Accounts Receivable Inventory (d) 500 (e) 300 (b)...
-
We have a 37.0 ( 0.5) wt% HCl solution with a density of 1.18 ( 0.01) g/mL. To deliver 0.050 0 mol of HCl requires 4.18 mL of solution. If the uncertainty that can be tolerated in 0.050 0 mol is 2%,...
-
Compute the molecular mass and its standard uncertainty for NH 3 . What is the percent relative uncertainty in molecular mass?
-
How many significant figures are there in the following numbers? (a) 1.903 0 (b) 0.039 10 (c) 1.40 10 4
-
4. Recall, the set of functions from a set A to a set B is denoted by BA. (a) Consider the set S = {a, b, c} and design a bijection between NS (the set of all functions from {a, b, c} to N) and the...
-
Exercise 4 (11 points). Let n be an even positive integer, and p be a prime such that p | n+1. Prove that p = 1 (mod 4). (Hint:) First, show that (H)-) =
-
Consider a retirement savings account where the monthly contribution is $125 for the first 25 years, is increased to $230 for the next 20 years, and then is increased once again to $475 for the last...

Study smarter with the SolutionInn App


