a. Show that at moderately low pressures and densities the virial equation of state can be written
Question:
a. Show that at moderately low pressures and densities the virial equation of state can be written as
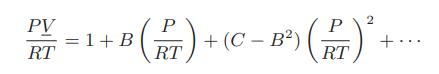
b. Prove that the fugacity coefficient for this form of the virial equation of state is
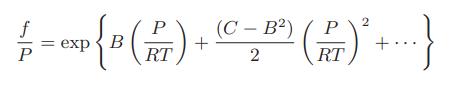
c. The first two virial coefficients for methyl fluoride at 50°C are B = −0.1663 m3 /kmol and C = 0.012 92 (m3 /kmol)2. Plot the ratio f /P as a function of pressure at 50°C for pressures up to 150 bar. Compare the results with the corresponding-states plot of f /P versus P/Pc.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:





