A thermally insulated (adiabatic) constant-volume bomb has been very carefully prepared so that half its volume is
Question:
A thermally insulated (adiabatic) constant-volume bomb has been very carefully prepared so that half its volume is filled with water vapor and half with subcooled liquid water, both at −10°C and 0.2876 kPa (the saturation pressure of the subcooled liquid). Find the temperature, pressure, and fraction of water in each phase after equilibrium has been established
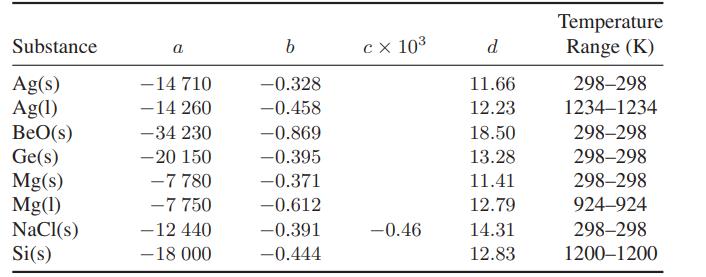
in the bomb. What is the entropy change for the process?
For simplicity, neglect the heat capacity of the bomb, assume the vapor phase is ideal, and for the limited temperature range of interest here, assume that each of the quantities below is independent of temperature.
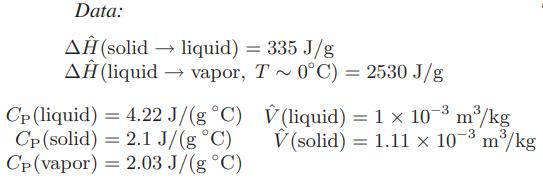
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:





