A well-insulated cylinder fitted with a frictionless piston initially contains nitrogen at 15C and 0.1 MPa. The
Question:
A well-insulated cylinder fitted with a frictionless piston initially contains nitrogen at 15°C and 0.1 MPa. The piston is placed such that the volume to the right of the piston is 0.5 m3, and there is negligible volume to the left of the piston and between the piston and the tank, which is also well insulated. Initially the tank of volume 0.25 m3 contains nitrogen at 400 kPa and 200°C. For the calculations below, assume that nitrogen is an ideal gas with C∗P = 29.3J/(mol K)
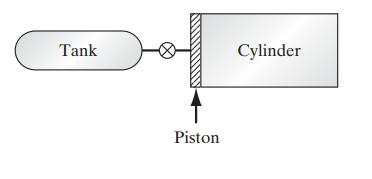
a. The piston is a perfect thermal insulator, and the valve between the cylinder and the tank is opened only long enough for the pressure in the tank and cylinder to equalize. What will the equalized pressure be, and what will be the temperatures of the nitrogen in the tank, in the cylinder to the left of the piston, and in the cylinder to the right of the piston?
b. What will the volumes in the cylinder be to the left and to the right of the piston?
c. How much entropy has been generated by the process?
Step by Step Answer:

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler





