Another possibility is to convert the methane directly to biomass that is used as animal feed. The
Question:
Another possibility is to convert the methane directly to biomass that is used as animal feed. The stoichiometry for this biochemical reaction is
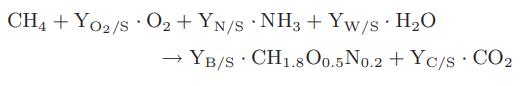
It has been found that the consumption of 2.5 m3 of methane (measured at 273 K and 1.013 bar) results in the production of 1 kg of biomass.
a. Determine all the yield factors for this reaction.
b. How much heat is released per m3 of methane consumed?
c. What fraction of the Gibbs energy of the methane is present in the biomass?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:





