At high temperaturesfor example, in a combustion processnitrogen and oxygen in air can react to form nitrous
Question:
At high temperatures—for example, in a combustion process—nitrogen and oxygen in air can react to form nitrous oxide,
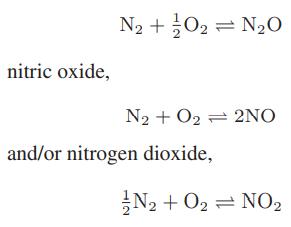
Starting with air (79 mol % nitrogen and 21 mol % oxygen), compute the equilibrium concentrations of all the oxides of nitrogen at atmospheric pressure over the temperature range from 1000 to 2000 K. [The oxides of nitrogen are referred to collectively as NOx compounds, and are smog-forming air pollutants.]
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:





