Calculate the molar volume, enthalpy, and entropy of carbon tetrachloride at 300C and 35 bar using the
Question:
Calculate the molar volume, enthalpy, and entropy of carbon tetrachloride at 300°C and 35 bar using the Peng-Robinson equation of state and the principle of corresponding states of Sec. 6.6. The following data are available:
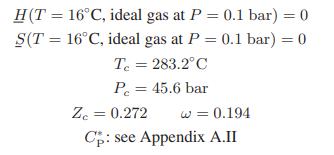
Appendix A.II
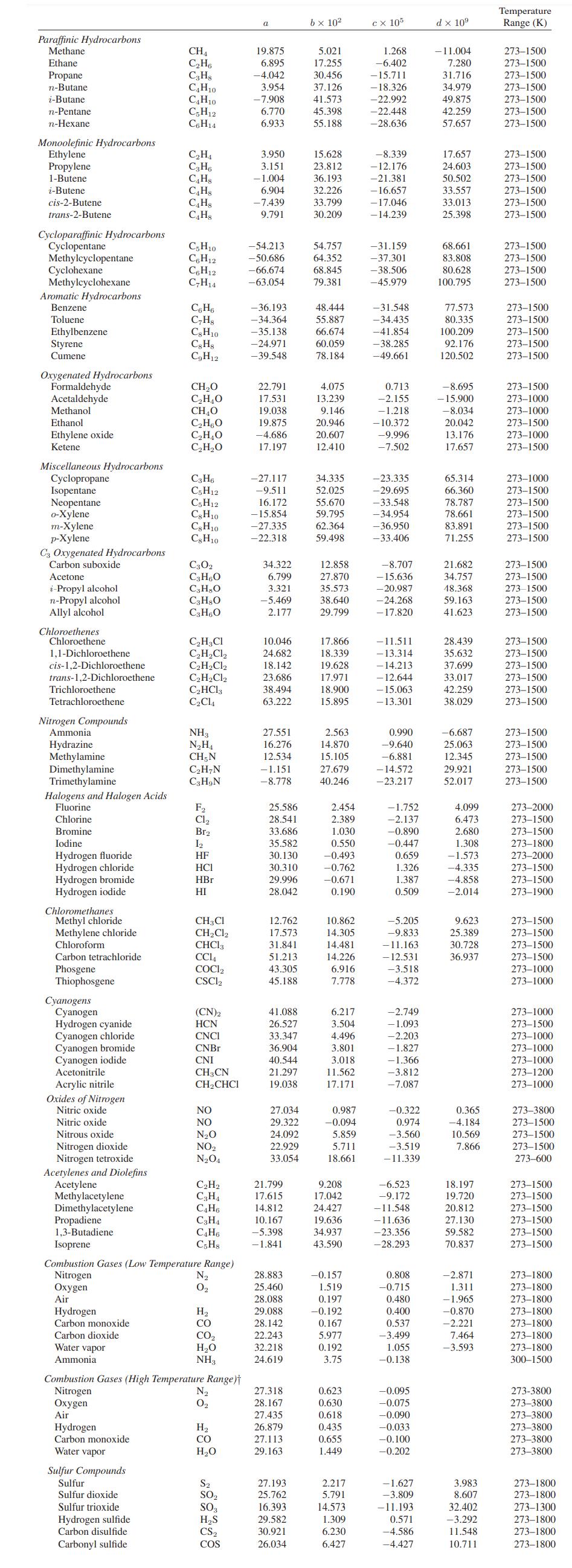
Transcribed Image Text:
H(T= 16°C, ideal gas at P = 0.1 bar) = 0 S(T 16°C, ideal gas at P = 0.1 bar) = 0 Te = 283.2°C P = 45.6 bar Zc = 0.272 w = 0.194 Cp: see Appendix A.II
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)

Answered By

Shem Ongek
I am a professional who has the highest levels of self-motivation. Additionally, I am always angled at ensuring that my clients get the best of the quality work possible within the deadline. Additionally, I write high quality business papers, generate quality feedback with more focus being on the accounting analysis. I additionally have helped various students here in the past with their research papers which made them move from the C grade to an A-grade. You can trust me 100% with your work and for sure I will handle your papers as if it were my assignment. That is the kind of professionalism that I swore to operate within. I think when rating the quality of my work, 98% of the students I work for always come back with more work which therefore makes me to be just the right person to handle your paper.
4.80+
174+ Reviews
426+ Question Solved
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:
Students also viewed these Engineering questions
-
Calculate the molar volume of saturated liquid and the molar volume of saturated vapor by the Redlich/Kwong equation for one of the following and compare results with values found by suitable...
-
The Double Dip Co. is expecting its ice cream sales to decline due to the increased interest in healthy eating. Thus, the company has announced that it will be reducing its annual dividend by 5% a...
-
The molar volume of a certain solid is 142.0 cm-1 mol-1 at 1.00 atm and 427.15 K, its melting temperature. The molar volume of the liquid at this temperature and pressure is 152.6 cm-1 mol-1. At 1.2...
-
A scholarship recipient may exclude from gross income the scholarship proceeds received for: Tuition, housing, and meals. O Tuition, books, and supplies. O Meals but not housing. O Meals and housing,...
-
The following are several independent events: 1. Change from the LIFO to the FIFO inventory cost flow assumption. 2. Reduction in remaining service life of machinery from 10 to 8 years. 3. A change...
-
Discuss each of the four fundamental issues for potential conflict during the project formation stage.
-
Can you create a graphic of the Fairmont payroll systems company expenses that highlight general accounting clerk, Mary Perez, has company expenses for FICA (social security), Medicare and 401K,...
-
Multiple Choice Questions The following questions deal with internal controls in the sales and collection cycle. Choose the best response. a. The accounting system will not post a sales transaction...
-
(5) A small maintenance project consists of the following jobs whose precedence relationships are given below: Job 1-2 1-3 2-3 2-5 3-4 Immediate Predecessor 15 15 3 5 8 8 Activity 3-6 4-5 4-6 5-6 6-7...
-
The force required to maintain a polymeric fiber at a length L when its unstretched length is L 0 has been observed to be related to its temperature by where is a positive constant. The heat...
-
Use Aspen Plus to compute the coefficient of performance of a Rankine cycle using water as the working fluid (described by the IAPWS-95 method) and the following state conditions: Condenser:...
-
Find the value of z/x at the point (1, 1, 1) if the equation xy + z 3 x - 2yz = 0 defines z as a function of the two independent variables x and y and the partial derivative exists.
-
5. A man pulls a crate of mass 10 kg with a constant speed. The crate is on frictionless bearings up a ramp that is h = 5 m high and angled at 30. (Hint: Remember the direction of force and...
-
A 12 g steel ball bearing rolls to the right at 68 cm/s and makes an elastic head-on collision with another steel ball bearing with a mass of 11 g that is initially at rest. After the collision, the...
-
6. A 16.00 m Three roller-coaster carts with a combined mass of 1250 kg have a speed of 15.0 m/s nearing position D. Ground B 8.50 m C D 2.50 m ODLB As the carts approach position D they are stopped...
-
Show that the total mechanical energy of a satellite (mass m) orbiting at a distance r from the center of the Earth (mass ME) is E = 1 GMME 2 r if U = 0 at r = o. (b) Show that although friction...
-
2. The mechanism shown in the figure consists of a particle m which is connected to a hinge A by an ideal rod with length . Particle m slides in a frictionless vertical slot of a block with mass M...
-
A 5-year, $1,000 par value, zero-coupon rate bond is to be issued to yield 10%. a. What should be the initial price of the bond? b. If immediately upon issue, interest rates dropped to 8%, what would...
-
Determine two different Hamilton circuits in each of the following graphs. A B F G
-
You are a new engineer working for a motorcycle manufacturer that produces a bike with a known control system instability. This instability can cause the rider to lose control at high speed and...
-
A mass of M = 1.0 kg is hung from a circular wire of diameter 0.20 mm as shown in Figure A. What is the stress in the wire? The following figures depict the situations described in above exercise....
-
If the wire in Figure A is stretched from 1.00 m to 1.01 m in length, what is the strain of the wire? The following figures depict the situations described in above exercise. Diameter 0.20 mm L Cube...
-
3. A sturdy ramp is an asymmetric 2-sided construction, with ramps going upward from either side. The overall 'double ramp' appears triangular from a 'side view' (see the diagram below). The ramp up...
-
Tesla, Inc. (NASDAQ:TSLA) CEO Elon Musk announced in March 2021 that Tesla vehicles can be purchased with bitcoin (BTC). Since corporate taxes must be paid in U.S. dollars (USD), Tesla is exposed to...
-
List the elements of a control loop. Do you have any hands-on experience? Tell me how to put a distillation system online. How do you find information about a company? What are your personal goals in...

Study smarter with the SolutionInn App


