How much entropy is generated per mole of chlorodifluoromethane that passes through the pressurereducing valve of the
Question:
How much entropy is generated per mole of chlorodifluoromethane that passes through the pressurereducing valve of the previous problem?
Data From Previous Probem
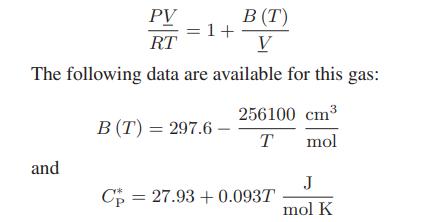
In a continuous manufacturing process, chlorodifluoromethane (CHClF2), initially at 10 bar and 420 K, passes through an adiabatic pressure-reducing valve so that its pressure drops to 0.1 bar (this last pressure low enough that CHClF2 can be considered to be an ideal gas). At these operating conditions, the gas can be represented by a one-term virial equation of state:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:





