Polar molecules interact more strongly at large distances than do nonpolar molecules, and generally form nonideal solutions.
Question:
Polar molecules interact more strongly at large distances than do nonpolar molecules, and generally form nonideal solutions. One model for solution nonidealities in a binary mixture consisting of a nonpolar species, which we denote by A, and a polar substance, designated by the symbol B, is based on the supposition that the polar substance partially dimerizes,
![]() Thus, although the mixture is considered to be a binary mixture with mole fractions
Thus, although the mixture is considered to be a binary mixture with mole fractions
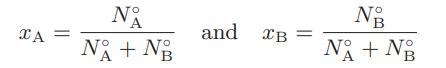
where N°A and N°B are the initial number of moles of A and B, respectively, it is, according to the supposition, really a ternary mixture with mole fractions
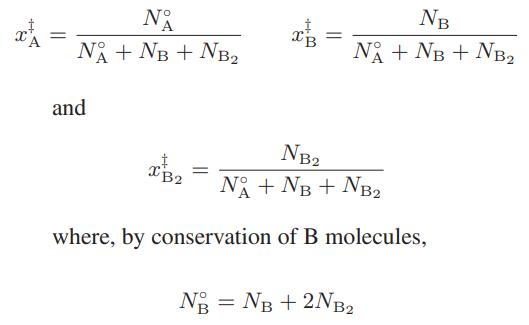
It is further assumed that the ternary mixture is ideal, and that the apparent nonidealities in mixture properties result from considering the A-B solution to be a binary mixture with mole fractions xi, rather than a ternary mixture with true fractions x‡i . Show that the apparent activity coefficients for the binary mixture that result from this model are
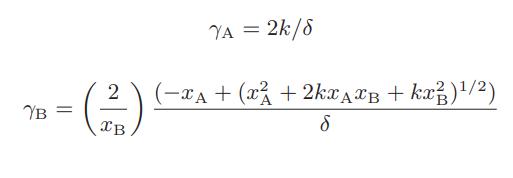
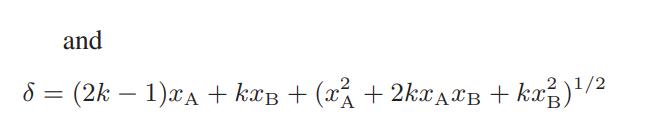
where k = 4Ka + 1, and Ka is the equilibrium constant for the dimerization reaction.
Step by Step Answer:

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler





