Redo Problem 13.25 using Aspen Plus. Problem 13.25 When pure hydrogen iodide gas enters an evacuated cylinder,
Question:
Redo Problem 13.25 using Aspen Plus.
Problem 13.25
When pure hydrogen iodide gas enters an evacuated cylinder, the following reactions may occur:
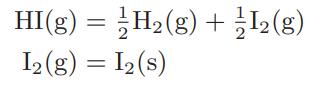
(Note that since Gibbs energies of formation data are available for iodine in both the gaseous and solid phases, it is more convenient to think of the solid-vapor iodine phase equilibrium as a chemical equilibrium.) If the reaction mixture is gradually compressed at 25°C, a pressure is reached at which the first bit of solid iodine appears. What is the pressure at which this occurs, and what is the vapor composition at this pressure?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:





