Styrene can be hydrogenated to ethyl benzene at moderate conditions in both the liquid phase and the
Question:
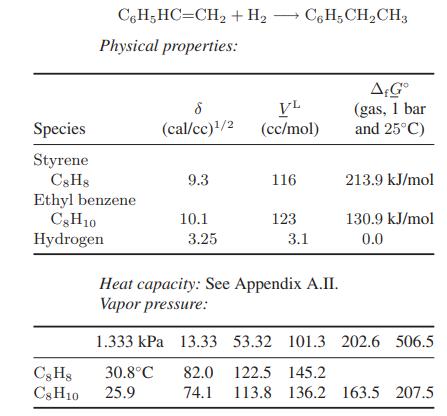
Styrene can be hydrogenated to ethyl benzene at moderate conditions in both the liquid phase and the gas phase. Calculate the equilibrium compositions in the vapor and liquid phases of hydrogen, styrene, and ethyl benzene at each of the following conditions:
a. 3-bar pressure and 25°C, with a starting mole ratio of hydrogen to styrene of 2 to 1
b. 3-bar pressure and 150°C, with a starting mole ratio of hydrogen to styrene of 2 to 1
Data:
Reaction stoichiometry:
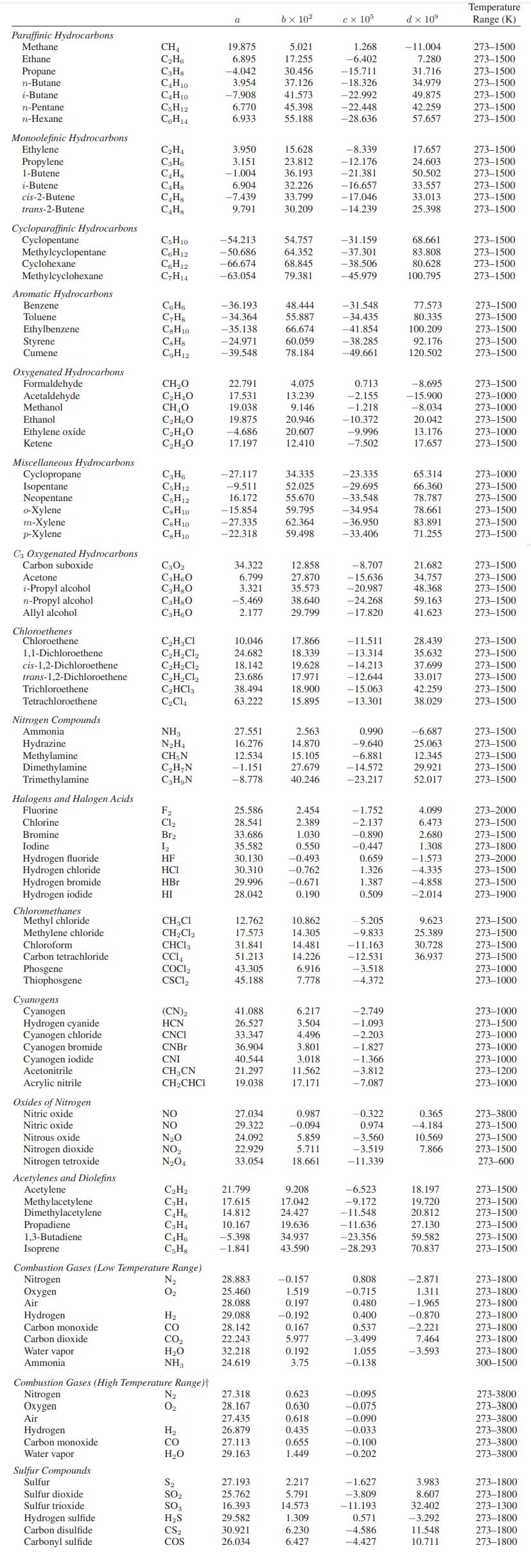
Appendix A.II.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:





