The effect of pressure on the melting temperature of solids depends on the heat of fusion and
Question:
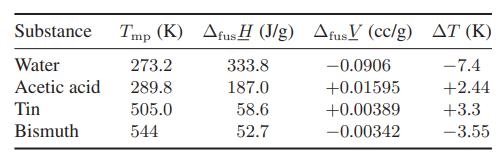
The effect of pressure on the melting temperature of solids depends on the heat of fusion and the volume change on melting. The heat of fusion is always positive (that is, heat must be added to melt the solid), while the volume change on melting will be positive if the solid is a close-packed structure, and negative for solids that crystallize into an open structure. Metallic elements generally crystallize into close-packed structures, so that ΔfusV is positive for most metals. Using the data below, calculate the change in the melting temperature Tmp for each substance for a pressure increase from 1 bar to 1001 bar, and compare the results with the experimentally
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:





