The following data are available for the infinitedilution activity coefficients in actetone in ethanol: a. Compute the
Question:
The following data are available for the infinitedilution activity coefficients in actetone in ethanol:
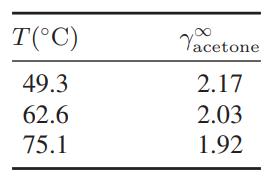
a. Compute the excess partial molar enthalpy of acetone in ethanol at 62.6°C.
b. Make a thermodynamically based estimate of the value of the infinite-dilution activity coefficient of acetone in ethanol at 100°C. (A simple linear extrapolation is not correct.)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:





