The following data are available for the solubility of barium sulfate in water: where values for the
Question:
The following data are available for the solubility of barium sulfate in water:
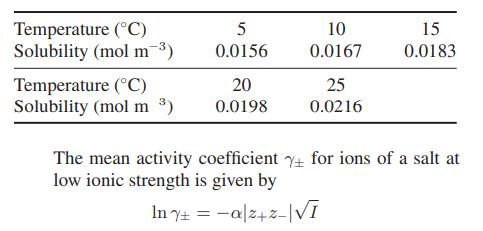
where values for the parameter α for water are given in Table 9.10-1.
a. Compute K°s , the ideal solution solubility product, for barium sulfate at each of the temperatures in the table.
b. At each of the temperatures in the table calculate the Gibbs energy change for the reaction
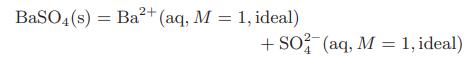
Here (s) denotes the pure solid state, and (aq, M = 1, ideal) indicates the ion in a hypothetical ideal aqueous solution at 1-molal concentration.
c. Compute the entropy and enthalpy changes for the reaction in part (b) at 5°C, 15°C, and 25°C.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:





