The following reaction occurs in air: for t in seconds and concentrations in kmol/m 3 . The
Question:
The following reaction occurs in air:
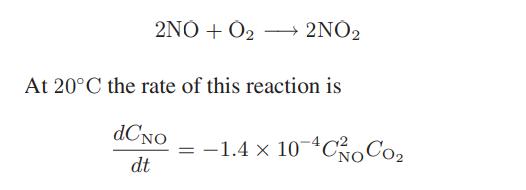
for t in seconds and concentrations in kmol/m3. The reaction occurs in a constant-volume, 2-L vessel, and the initial concentration of NO is 1 kmol/m3 and that of O2 is 3 kmol/m3
a. If 0.5 mol of NO reacts, how much NO2 is produced?
b. Determine how long it would take for 0.5 mol of NO to have reacted.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:





