The piston-and-cylinder device shown here contains an ideal gas at 20 bar and 25C. The piston has
Question:
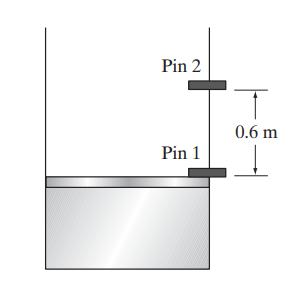
The piston-and-cylinder device shown here contains an ideal gas at 20 bar and 25◦C. The piston has a mass of 300 kg and a cross-sectional area of 0.05 m2. The initial volume of the gas in the cylinder is 0.03 m3, the piston is initially held in place by a pin, and the external pressure on the piston and cylinder is 1 bar. The pin suddenly breaks, and the piston moves 0.6 m farther up the cylinder, where it is stopped by another pin. Assuming that the gas is ideal with a constant-pressure heat capacity of 30 J/(mol K), and that there is no heat transfer between the gas and the cylinder walls or piston, estimate the piston velocity, and the temperature and pressure of the gas just before the piston hits the second pin. Do this calculation assuming
a. No friction between the piston and the cylinder
b. Friction between the piston and the cylinder
List and defend all assumptions you make in solving this problem.
Step by Step Answer:

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler





