Very polar molecules may associate in the gas phase. One example is acetic acid, which, because of
Question:
Very polar molecules may associate in the gas phase. One example is acetic acid, which, because of its structure, can form dimers but not higher polymers. For the reaction
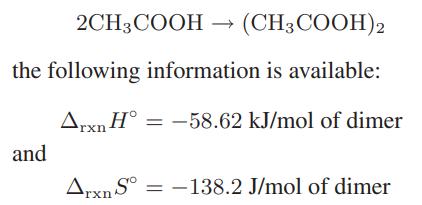
These values are for the ideal gas, 1 bar standard state, and can be assumed to be independent of temperature.
a. Compute the degree of dimerization of acetic acid at 25°C and at 0.1, 1, and 10 bar.
b. Compute the degree of dimerization of acetic acid at 100°C and at 0.1, 1, and 10 bar.
c. If one assumes that only the monomer is present, acetic acid does not satisfy the ideal gas law. However, it is thought that at the temperatures and pressures considered above, the acetic acid monomer and dimer form an equilibrium ideal gas mixture, and that the apparent nonideality that is found when only monomer is assumed to be present is actually the result of the mole number change due to dimerization. Develop an equation of state for acetic acid that takes into account dimerization but has temperature, pressure, volume, and the number of moles of acetic acid if no dimerization occurred as the independent variables.
Step by Step Answer:

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler





