While ethanol can be made by biological fermentation, for large-scale production the following nonbiological reaction starting with
Question:
While ethanol can be made by biological fermentation, for large-scale production the following nonbiological reaction starting with ethylene and water can be used instead:
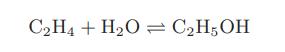
If stoichiometric amounts of ethylene and water are used, compute the equilibrium constant and the equilibrium extent of reaction at
a. 1 bar and 25°C, assuming the liquid solution is ideal
b. Repeat the calculation assuming a nonideal liquid solution if formed.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:





