A chronoamperometry experiment was performed on a (4.00 mathrm{mM}) solution of an iron(III) complex at (25^{circ} mathrm{C})
Question:
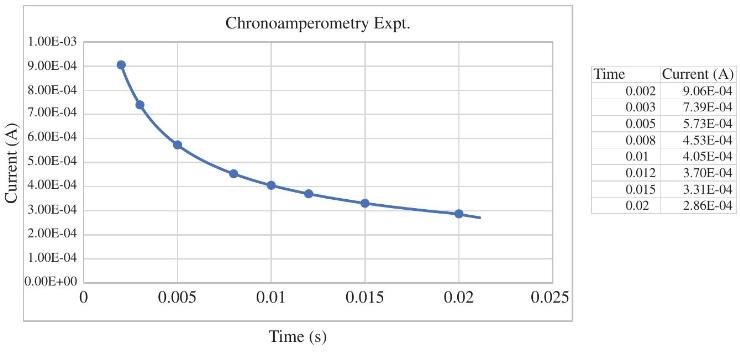
A chronoamperometry experiment was performed on a \(4.00 \mathrm{mM}\) solution of an iron(III) complex at \(25^{\circ} \mathrm{C}\) using a platinum electrode with an area of \(0.075 \mathrm{~cm}^{2}\). The potential was stepped from a voltage that was \(100 \mathrm{mV}\) more positive than the \(\mathrm{E}^{\circ ´}\) out to a value that was \(100 \mathrm{mV}\) more negative than \(\mathrm{E}^{\circ ´}\) where a one-electron reduction occurs. The data for the current-time curve are given below. Calculate the diffusion coefficient for the iron(III) complex under these conditions.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Electroanalytical Chemistry Principles Best Practices And Case Studies
ISBN: 9781119538592,9781119538585
1st Edition
Authors: Gary A. Mabbott
Question Posted:





