A saturated aqueous solution of sodium chloride at (100^{circ} mathrm{C}) is fed to a steady-state evaporative crystallizer
Question:
A saturated aqueous solution of sodium chloride at \(100^{\circ} \mathrm{C}\) is fed to a steady-state evaporative crystallizer system that operates at \(100^{\circ} \mathrm{C} . \mathrm{F}_{\mathrm{W}, \text { in }}=2000 \mathrm{~kg} / \mathrm{h}\) of water(on a saltfree basis). We evaporate \(\mathrm{V} \mathrm{kg} / \mathrm{h}\) of pure water vapor. The product mixture of crystals and solution at \(100^{\circ} \mathrm{C}\) from the crystallizer is sent to a solid-liquid separator. The solid crystals plus entrained saturated liquid are removed as product that is sent to a drier and the remainder of the saturated liquid is recycled. Volumetric entrainment of saturated liquid with the solid salt can be approximated as \(\mathrm{e} \times\) (volume solid salt) with \(\mathrm{e}=0.2\). Solubility data are in Table 17-1. Additional data are in Problem 15.D23. Determine flow rates in \(\mathrm{kg} / \mathrm{h}\) of V, A (anhydrous crystals), and entrained saturated solution.
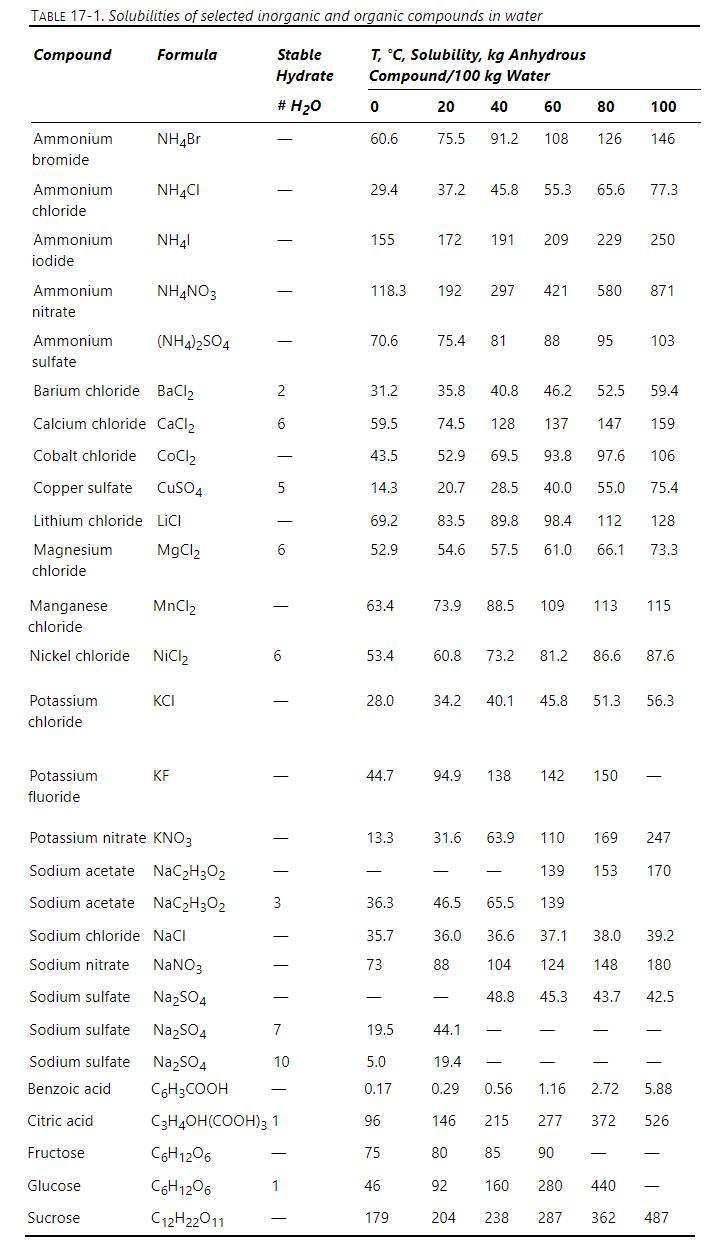
Data From Problem D23.
A saturated sodium nitrate, \(\mathrm{NaNO}_{3}\), solution at \(100^{\circ} \mathrm{C}\) at a feed rate of \(1000 \mathrm{~kg} / \mathrm{h}\) of water is sent to a steady-state evaporative crystallizer also operating at \(100^{\circ} \mathrm{C}\). The evaporative crystallizer evaporates \(300 \mathrm{~kg} / \mathrm{h}\) of pure water vapor. The mixture of the liquid and crystals from the evaporative crystallizer is cooled to \(35^{\circ} \mathrm{C}\) and sent to a steady-state cooling crystallizer also operating at \(35^{\circ} \mathrm{C}\). Data are in Table \(17-1\).
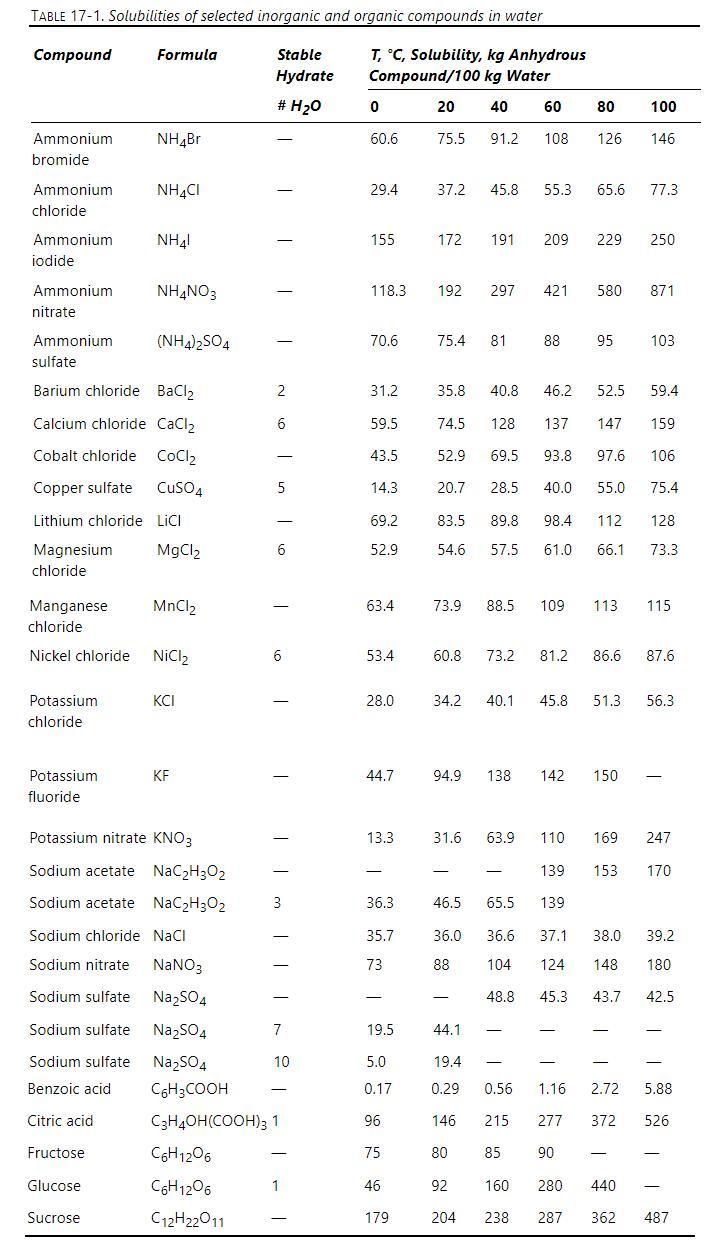
a. How many \(\mathrm{kg}\) of water, how many \(\mathrm{kg}\) of crystals, and how many \(\mathrm{kg}\) of dissolved \(\mathrm{NaNO}_{3}\) are in the product from the evaporative crystallizer?
b. How many \(\mathrm{kg}\) of water, how many \(\mathrm{kg}\) of crystals, and how many \(\mathrm{kg}\) of dissolved \(\mathrm{NaNO}_{3}\) are in the product from the cooling crystallizer?
Step by Step Answer:

Separation Process Engineering Includes Mass Transfer Analysis
ISBN: 9780137468041
5th Edition
Authors: Phillip Wankat





