A simple batch distillation (Figure 9-1) is separating (8.00 mathrm{kmol}) of a feed that is (40.0 mathrm{~mol}
Question:
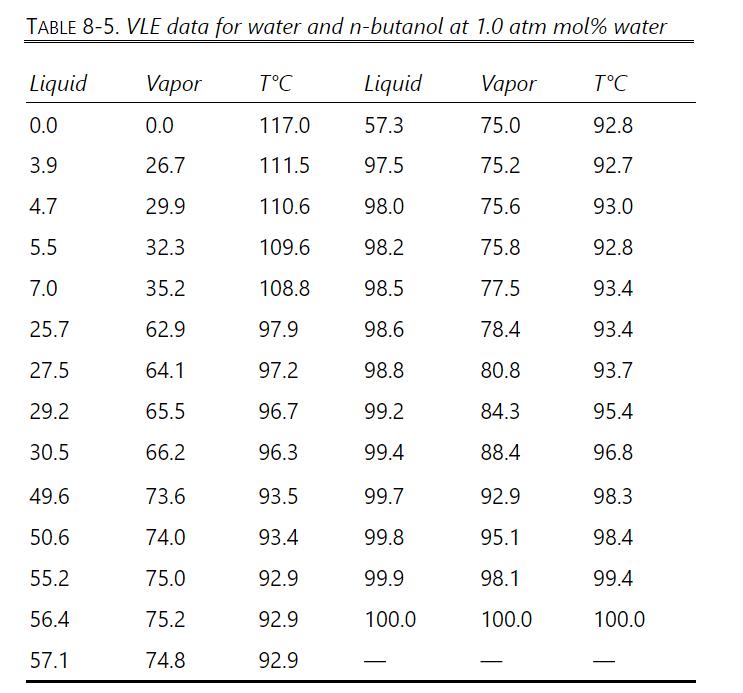
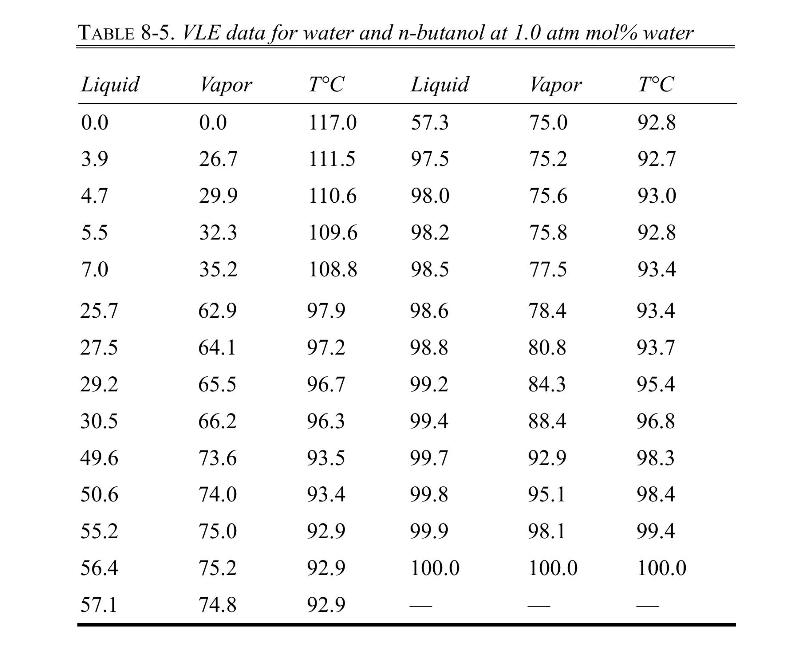
A simple batch distillation (Figure 9-1) is separating \(8.00 \mathrm{kmol}\) of a feed that is \(40.0 \mathrm{~mol} \%\) water and \(60.0 \mathrm{~mol} \% \mathrm{n}\)-butanol. The batch distillation is continued until the still pot contains 0.080 mole fraction water. VLE data are in Table 8-5, Problem 8.D3.
Figure 9-1
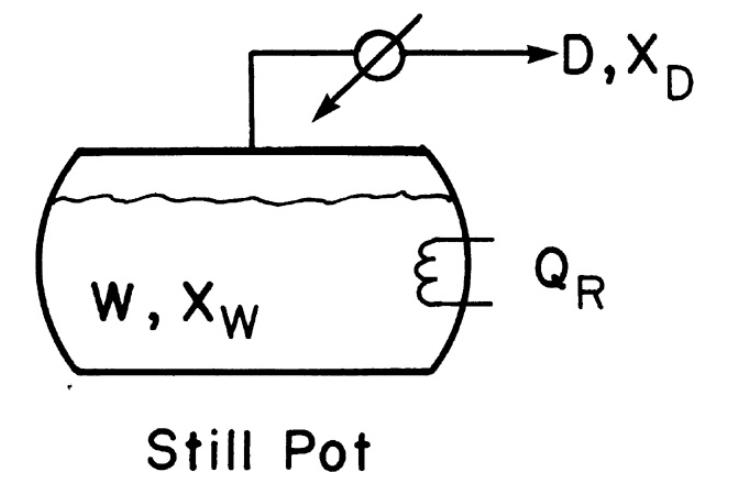
Table 8-5
Problem 8.D3
VLE data for water and n-butanol are given in Table 8-5. We have flash distillation systems separating \(100.0 \mathrm{kmol} / \mathrm{h}\) of two different water and \(\mathrm{n}-\) butanol mixtures.
a. Find \(\mathrm{W}_{\text {final }}, \mathrm{D}_{\text {total }}\), and \(\mathrm{x}_{\mathrm{D}, \text { avg }}\).
b. After settling, the final distillate product is two liquid phases. What are the mole fractions and the amounts (kmol) of each liquid phase?
Comparison with the solution to Problem 9.D13 shows that Figure \(9-10\) gives a better separation.
Problem 9.D13
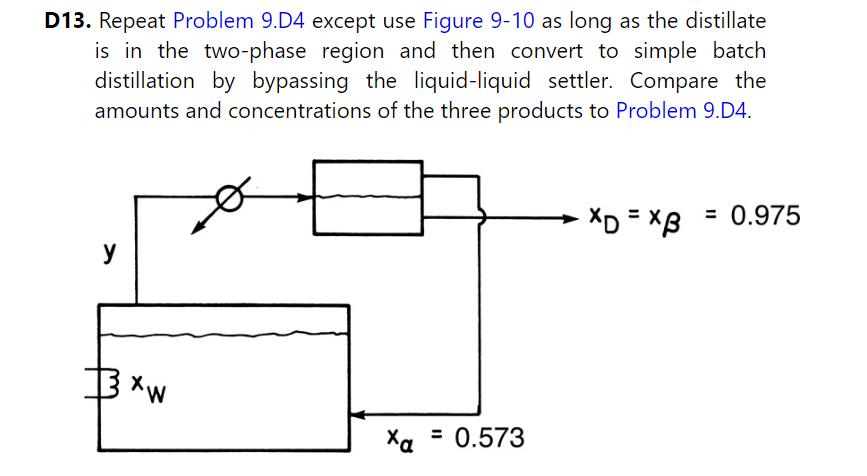
Step by Step Answer:

Separation Process Engineering Includes Mass Transfer Analysis
ISBN: 9780137468041
5th Edition
Authors: Phillip Wankat





