For extraction of benzoic acid from water into toluene with toluene the dispersed phase, we measure the
Question:
For extraction of benzoic acid from water into toluene with toluene the dispersed phase, we measure the following mole fractions of benzoic acid: \(\mathrm{x}_{\mathrm{D}, \text { in }}=0, \mathrm{x}_{\mathrm{D}, \text { out }}=0.00023\), and \(\mathrm{x}_{\mathrm{C}, \text { out }}=1.99 \times 10^{-6}\). The mixer is \(0.75 \mathrm{~m}\) tall and has a \(0.75 \mathrm{~m}\) diameter. Flow rate of the dispersed phase is \(\mathrm{Q}_{\mathrm{D}}=0.0012 \mathrm{~m}^{3} / \mathrm{s}\) and \(\mathrm{Q}_{\mathrm{C}}=0.097 \mathrm{~m}^{3} / \mathrm{s}\). Data for density, equilibrium, and molecular weight are in Example 16-5.

a. Determine stage efficiency \(\mathrm{E}_{\mathrm{MD}, \text { mole_frac }}\). Note: The unit conversion in Eq. (16-94) is required to calculate \(\mathrm{m}\) for equilibrium, \(\mathrm{y}=\mathrm{mx}\), in mole fractions.
Eq.16-94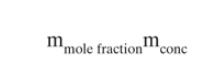 b. Calculate value of \(\mathrm{K}_{\mathrm{O}-\mathrm{ED}} \mathrm{a}\) using the completely mixed staged model.
b. Calculate value of \(\mathrm{K}_{\mathrm{O}-\mathrm{ED}} \mathrm{a}\) using the completely mixed staged model.
c. Calculate value of \(\mathrm{K}_{\mathrm{O}-\mathrm{ED}} \mathrm{a}\) using the differential equation model.
d. If by accident the value of KO−EDa calculated using the completely mixed staged model is used to predict the stage efficiency using the differential equation model, what (incorrect) value of EMD,mole_frac is obtained?
Step by Step Answer:

Separation Process Engineering Includes Mass Transfer Analysis
ISBN: 9780137468041
5th Edition
Authors: Phillip Wankat





