Generalize your result to Problem 7.7 as follows: A first-order reaction is to be carried out in
Question:
Generalize your result to Problem 7.7 as follows: A first-order reaction is to be carried out in a batch reactor and in a CFSTR to achieve a relative reduction RR in concentration. (In Problem 7.7, RR = 100.) Compare the required time tB in a batch reactor to the required residence time θ in a CFSTR to achieve the same RR.
Problem 7.7
The decomposition of the carcinogen N-nitrosodiethylamine (NDEA) in water was shown in Example 6.3 to be first order when exposed to ultraviolet radiation at an intensity of 1 mW/cm2, with a rate constant 0.59 min−1.
a. What is the time required in a batch reactor to decrease the concentration in a wastewater sample by a factor of 100?
b. What is the residence time required in a CFSTR to achieve the same reduction?
Example 6.3:
N-nitrosodiethylamine (NDEA) is a carcinogenic compound that is found in drinking water, together with other nitrosamines. One way in which NDEA can be broken down is by exposure to ultraviolet radiation. The data in Table 6.3 were read from a published graph reporting on an experiment inwhich 0.10 mmol/L of NDEA at pH 6.0 was exposed to 1 mW/cm2 of ultraviolet radiation. Are the data consistent with first-order kinetics?
Table 6.3:
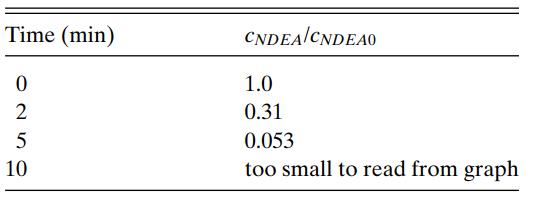
The data are plotted as ln(cNDEA/cNDEA0) versus time in Figure 6.5.The available data points lie on a straight line passing through the origin with a slope of −0.59 min −1 . Hence, according to Equation 6.13, the limited available data are consistent with first-order or pseudo-first-order kinetics.
Figure 6.5:
Step by Step Answer:





