If for a cyclic voltammogram the (E^{mathrm{O} prime}) for quinone, (Q), appears at a (+0.220 mathrm{~V}) at
Question:
If for a cyclic voltammogram the \(E^{\mathrm{O} \prime}\) for quinone, \(Q\), appears at a \(+0.220 \mathrm{~V}\) at a platinum electrode (versus silver/silver chloride ref. electrode) at \(\mathrm{pH} 7.0\), what direction would \(E^{\circ \prime}\) shift at \(\mathrm{pH}6.0\) ?
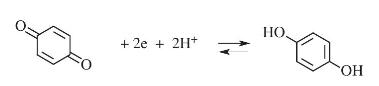
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Electroanalytical Chemistry Principles Best Practices And Case Studies
ISBN: 9781119538592,9781119538585
1st Edition
Authors: Gary A. Mabbott
Question Posted:





