In Problem (15 . mathrm{H} 5) changing the pressure changes the diffusivities but does not change the
Question:
In Problem \(15 . \mathrm{H} 5\) changing the pressure changes the diffusivities but does not change the Henry's law constant of ammonia. However, changing the pressure does change the surface concentrations. Explain.
Problem 15.H5
Repeat Example \(15-8\) but for a pressure of \(1.1 \mathrm{~atm}\). The diffusivities depend on pressure. Also answer Problem 15.A6.
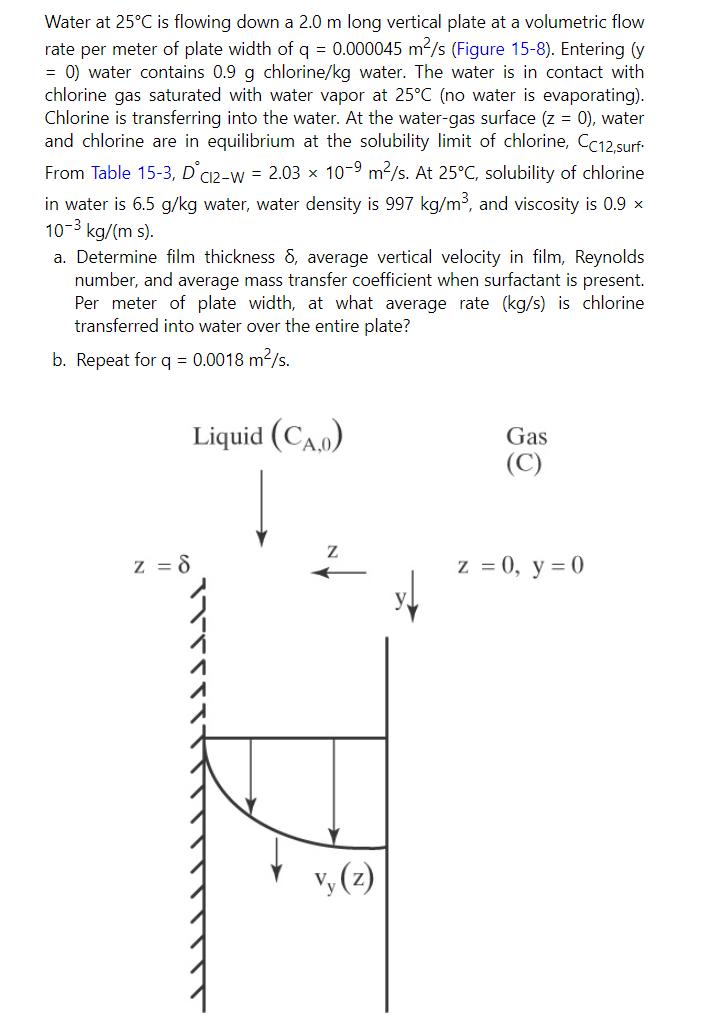
Example 15-8
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Separation Process Engineering Includes Mass Transfer Analysis
ISBN: 9780137468041
5th Edition
Authors: Phillip Wankat
Question Posted:





