Many extraction systems are partially miscible at high concentrations of solute but close to immiscible at low
Question:
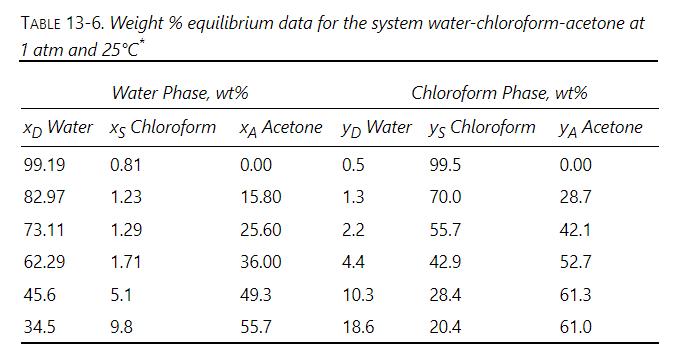
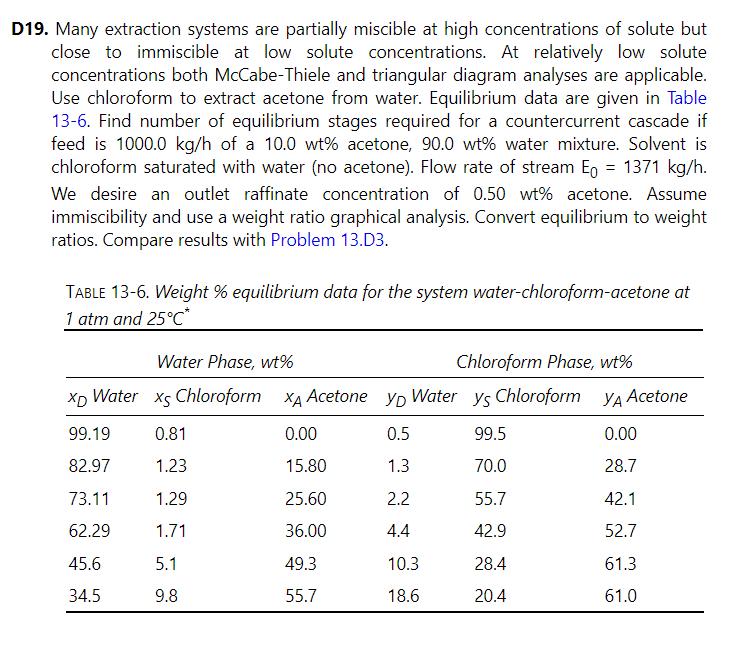
Many extraction systems are partially miscible at high concentrations of solute but close to immiscible at low solute concentrations. At relatively low solute concentrations both McCabe-Thiele and triangular diagram analyses are applicable. Use chloroform to extract acetone from water. Equilibrium data are given in Table 13-6. Find number of equilibrium stages required for a countercurrent cascade if feed is \(1000.0 \mathrm{~kg} / \mathrm{h}\) of a \(10.0 \mathrm{wt} \%\) acetone, \(90.0 \mathrm{wt} \%\) water mixture. Solvent is chloroform saturated with water (no acetone). Flow rate of stream \(\mathrm{E}_{0}=1371 \mathrm{~kg} / \mathrm{h}\). We desire an outlet raffinate concentration of \(0.50 \mathrm{wt} \%\) acetone. Assume immiscibility and use a weight ratio graphical analysis. Convert equilibrium to weight ratios. Compare results with Problem 13.D3.
Problem 13.D3.
Use chloroform to extract acetone from water. Equilibrium data are given in Table 13-6. Find number of equilibrium stages required for a countercurrent cascade if feed is \(1000.0 \mathrm{~kg} / \mathrm{h}\) of a \(10.0 \mathrm{wt} \%\) acetone, \(90.0 \mathrm{wt} \%\) water mixture. Solvent is chloroform saturated with water (no acetone). Flow rate of stream \(E_{0}=1371 \mathrm{~kg} / \mathrm{h}\). Outlet raffinate concentration is \(0.50 \mathrm{wt} \%\) acetone. Use a triangle diagram and compare results with Problem 13.D19.
Problem 13.D19
Step by Step Answer:

Separation Process Engineering Includes Mass Transfer Analysis
ISBN: 9780137468041
5th Edition
Authors: Phillip Wankat





