The catalytic gas phase oxidation of naphthalene to form phthalic anhydride is thought to take place as
Question:
The catalytic gas phase oxidation of naphthalene to form phthalic anhydride is thought to take place as follows: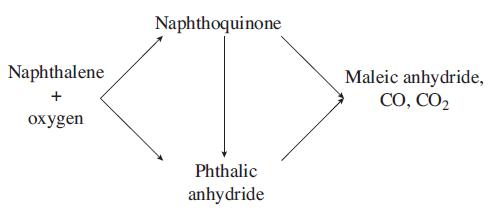
The reaction has been studied by DeMaria, Longfield, and Butler [Ind. Eng. Chem., 53, 259 (1961)] in a fluidized bed reactor with two catalysts, designated A and B. A surprisingly good way to model a fluidized bed reactor is to assume that it behaves like a CFSTR, using the same equations as for a liquid-phase system. When oxygen is in excess, which is always the case in commercial operation, the reaction is well described by the simple consecutive scheme naphthalene (A)→phthalic anhydride (M) → undesired combustion products (S), where both reactions are pseudo-firstorder and irreversible. Let k1 = k10 exp(−E1/RT) be the first-order rate constant for A → M and k2 = k20 exp(−E2/RT) be the first-order rate constant for M → S. The following data are available:
a. Compute the selectivity s = cR/(cAf − cA) and the yield y = cR/cAf in a reactor with a residence θ = V/q of one second (1s) and plot the results for a range of temperatures between 224 and 500◦C.
b. Select the reactor operating temperature at the point where you estimate that there is a maximum in the yield curve. Pick two residence times that are different from 1s and see if you can improve the yield. Which catalyst is more efficient?
c. How does the selectivity affect the reactor design? Are there differences between catalysts A and B?
d. How would you select the optimal residence time for the phthalic anhydride reaction?
Step by Step Answer:





