The data in Table 6.P3 are for reactant A in the irreversible reaction A + B
Question:
The data in Table 6.P3 are for reactant A in the irreversible reaction A + βB → products. The initial concentrations are believed to be in stoichiometric balance. Estimate the reaction order and find the rate constant.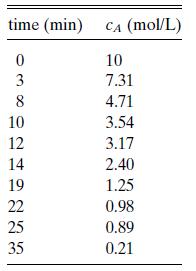
Transcribed Image Text:
time (min) CA (mol/L) 0 10 3 7.31 8 4.71 3.54 3.17 2.40 1.25 10 12 14 19 22 25 35 0.98 0.89 0.21
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)

Answered By

FREDRICK MUSYOKI
Professional Qualities:
Solution-oriented.
Self-motivated.
Excellent problem-solving and critical thinking skills.
Good organization, time management and prioritization.
Efficient troubleshooting abilities.
Tutoring Qualities:
I appreciate students as individuals.
I am used to tailoring resources for individual needs.
I can integrate IT into student's lessons.
I am good at explaining concepts.
I am able to help students progress.
I have a wide curriculum knowledge.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
The Tastee Bakery Company supplies a bakery product to many supermarkets in a metropolitan area. The company wishes to study the effect of the height of the shelf display employed by the supermarkets...
-
A disproportionation reaction occurs when a single reactant is both oxidized and reduced. Balance and determine the voltage of this disproportionation reaction. Use the data in Table 14.1. Cr2+ ( Cr...
-
A disproportionation reaction occurs when a single reactant is both oxidized and reduced. Balance and determine the voltage of this disproportionation reaction. Use the data in Table 14.1. Fe2+ ( Fe...
-
Mick Stone disposed of the following assets during tax year 2020-21: (1) On 19 May 2020, Mick sold a freehold warehouse for 522,000. This warehouse was purchased on 6 August 2008 for 258,000, and was...
-
In recent years the stock market experienced significant declines, unprecedented since the Great Depression. Why might it be important for you to consider current economic events as part of planning...
-
From August 2009 to August 2015, the Standard & Poor's Index of 500 stock prices more than doubled, while the consumer price index increased by just over 10 percent. Briefly explain what effect, if...
-
Draw a frequency histogram for the frequency distribution in Example 2. Describe any patterns. Data from Example 2 Using the frequency distribution constructed in Example 1, find the midpoint,...
-
An Object at an Angle A 16.0-cm-long pencil is placed at a 45.0? angle, with its center 15.0 cm above the optic axis and 45.0 cm from a lens with a 20.0-cm focal length as shown in Fig. 34.65. (Note...
-
1. From the HRM perspective, what are the implications of managing labor relations in the Public Sector? 2. Discuss whether unions are still relevant and necessary in today's work environment. What...
-
The reaction ClO 3 + 3H 2 SO 4 Cl + 3SO 4 = + 6H + in 0.2N H 2 SO 4 was studied in 1932 by Nixon and Krauskopf, who reported the data in Table 6.P2. Find a rate expression that is consistent with...
-
Reconsider the data in Table 6.1 for the reaction between sulfuric acid and diethyl sulfate, and suppose that you believe that the forward reaction rate is actually of the form r = kc A . Test this...
-
Explain the concepts of restricted and unrestricted sums of squared errors and how they are used to test hypotheses.
-
Visit Cinnaholic to learn more about the company. Review Module 4, Unit 4 - How to Create a Customer Persona. Create a report for the following components: Identify and create a Marketing Mix (4Ps)...
-
Cinemark Holdings, Incorporated, operates movies and food concession counters throughout the United States. Its income statement for the quarter ended June 30, 2019, reported the following (accounts...
-
Jake's Roof Repair has provided the following data concerning its costs: Fixed cost per Month Cost per Repair Hour Wages and salaries $ 2 0 , 8 0 0 $ 1 5 . 0 0 Parts and supplies $ 7 . 7 0 Equipment...
-
Prepare journal entries to record each of the following sales transactions of a merchandising company. The company uses a perpetual inventory system and the gross method. April 1 Sold merchandise for...
-
Tech Solutions is a consulting firm that uses a job - order costing system. Its direct materials consist of hardware and software that it purchases and installs on behalf of its clients. The firm s...
-
What measures can companies can take to prevent and detect fraudulent electronic payments?
-
Suppose the concentration of glucose inside a cell is 0.1 mm and the cell is suspended in a glucose solution of 0.01 mm. a. What would be the free energy change involved in transporting 10-o mole of...
-
An electrically heated glove consists of a flat thin-sheet electrode embedded within a 0.01 m layer of an insulating material with a thermal conductivity of 0.02 W/m K, which is directly in contact...
-
A cube, 1.0-in. long on each side, is constructed of equal-sized, alternating parallel layers of copper and Pyrex glass. There are five layers of copper and five layers of glass. A question has...
-
The proposed design of a fuel element for a nuclear power reactor consists of 2-in.-diameter cylindrical fuel core surrounded by a 0.25-in.- thick aluminum-alloy cladding. The outside surface of the...
-
rojected benefit obligation ccumulated benefit obligation air value of plan assets $2,721,600 1,981,200 2,282,900 ccumulated OCI (PSC) 209,800 ccumulated OCI-Net loss (1/1/25 balance, 0) 45,300...
-
Sorra Corporation's records included the following shareholders' equity accounts: shares Preference Shares, par value P150, authorized 20,000 Share Premium-Preference Ordinary Shares, no-par, P50...
-
Sales revenue Coolbrook Company has the following information available for the past year: River Division $ 1,202,000 887,000 $ 315,000 $ 1,070,000 Stream Division $ 1,804,000 1,294,000 $ 510,000 $...

Study smarter with the SolutionInn App


