The following data were obtained by preparing each standard solution by diluting the appropriate volume of a
Question:
The following data were obtained by preparing each standard solution by diluting the appropriate volume of a \(1.00 \times 10^{-2} \mathrm{M} \mathrm{Cl}^{-}\)stock solution into \(250 \mathrm{ml}\) volumetric flasks and diluting to the mark with deionized water. Before measuring, \(100.0 \mathrm{ml}\) of each standard was spiked with \(1.00 \mathrm{ml}\) of \(4 \mathrm{M} \mathrm{NaNO}_{3}\) to adjust the ionic strength.
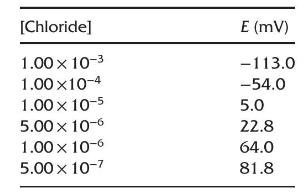
(a) What concentration of chloride ion would be indicated for a sample that was also treated with ionic strength buffer and gave a signal of \(15.5 \mathrm{mV}\) ?
(b) If the sample had not been treated with \(\mathrm{NaNO}_{3}\) to adjust the ionic strength before measuring, what would the potential reading have been (neglecting the influence on the junction potential for the reference bridge)?
Step by Step Answer:

Electroanalytical Chemistry Principles Best Practices And Case Studies
ISBN: 9781119538592,9781119538585
1st Edition
Authors: Gary A. Mabbott





