We have a flash drum separating (50.0 mathrm{kmol} / mathrm{h}) of a mixture of ethane, isobutane, =
Question:
We have a flash drum separating \(50.0 \mathrm{kmol} / \mathrm{h}\) of a mixture of ethane, isobutane, = and n-butane. The ratio of isobutane to n-butane is held constant at 0.80 (that is, Zic4/ZnC4 0.80). The mole fractions of all three components in the feed can change. If the flash drum operates at a pressure of 100.0 kPa, a temperature of 20.0C, and V/F = 0.40, what are the mole fractions of all three components in the feed? Use DePriester charts or Eq. (2-28).
Equations (2-28)
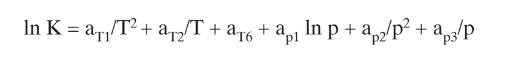
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Separation Process Engineering Includes Mass Transfer Analysis
ISBN: 9780137468041
5th Edition
Authors: Phillip Wankat
Question Posted:





