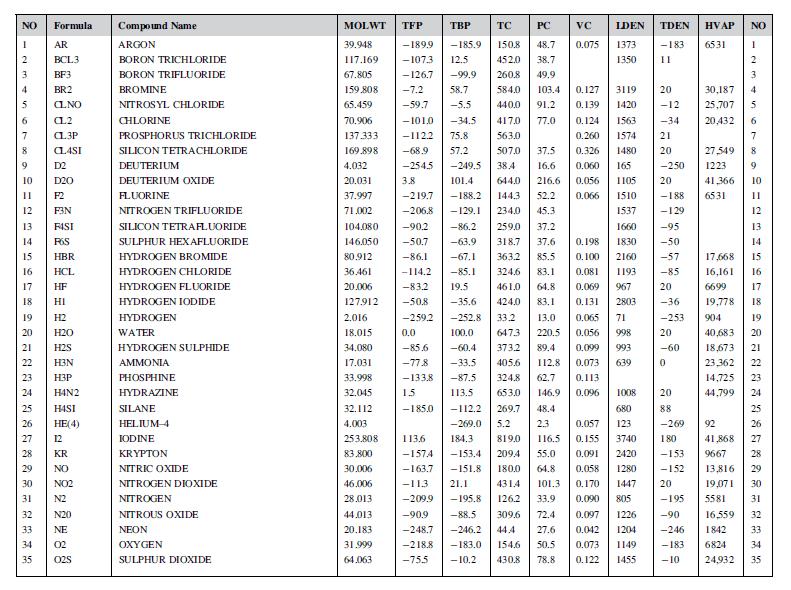
A gas produced as a by-product from the carbonization of coal has the following composition, mole per
Question:
A gas produced as a by-product from the carbonization of coal has the following composition, mole per cent: carbon dioxide 4, carbon monoxide 15, hydrogen 50, methane 12, ethane 2, ethylene 4, benzene 2, balance nitrogen. Using the data given in Appendix C, calculate the gross and net calorific values of the gas. Give your answer in MJ/m3, at standard temperature and pressure.
Data from appendix C
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Engineering Design
ISBN: 9780081025994
6th Edition
Authors: Ray Sinnott, R.K. Sinnott, Sinnott Gavin Towler
Question Posted:





