A healthcare company decides to market a hand warmer. It is basically a palm-size plastic pouch containing
Question:
A healthcare company decides to market a hand warmer. It is basically a palm-size plastic pouch containing a supersaturated aqueous salt solution; that is, the amount of dissolved salt exceeds its solubility limit. The degree of supersaturation is the ratio of the mass of dissolved salt per unit mass of water to the mass of salt at saturation; it is expected to be in the range of 1 to 2 . By flexing a small metal disk in the solution, the salt begins to crystallize, and the heat of crystallization produces a temperature rise rapidly. The amount of heat is sufficient to keep the hands holding the pouch warm over a period of up to \(30 \mathrm{~min}\). This hand warmer can be recharged by redissolving the salt in a microwave oven or in hot water.
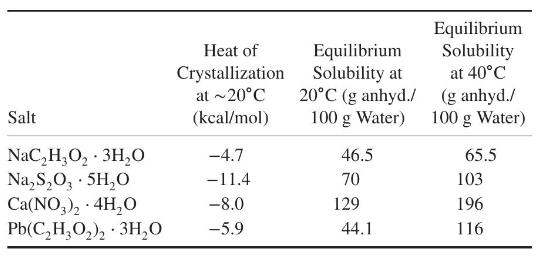
From the patent literature, four potential candidates can be identified: sodium acetate, sodium thiosulfate, calcium nitrate, and lead acetate. These are all hydrated at ambient temperatures with high solubility and relatively high heats of crystallization (Mullin, 1993).
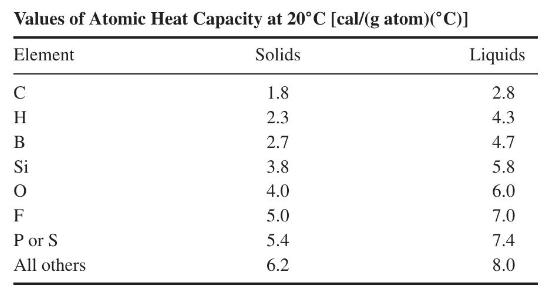
Data for the specific heat of these salts are not readily available but can be estimated using Kopp's rule, which states that the heat capacity of a solid compound or the compound in liquid form is approximately equal to the sum of the heat capacities of the individual atoms. The following table shows values for individual atoms determined from experimental data.
(a) The product performance calls for a temperature rise from \(20^{\circ} \mathrm{C}\) to \(40^{\circ} \mathrm{C}\). If all four salts can be assumed to be able to accommodate a supersaturation of up to around 2 , select one and justify its use in the hand warmer product. [Hint: Perform energy balance to determine the required amount of salt in a supersaturated solution.]
(b) Determine the product specifications. Specifically, provide the dimensions of the pouch, the amount of salt and water within the pouch, and the amount of heat available for hand warming.
Step by Step Answer:

Product And Process Design Principles Synthesis Analysis And Evaluation
ISBN: 9781119355243
4th Edition
Authors: Warren D. Seider, Daniel R. Lewin, J. D. Seader, Soemantri Widagdo, Rafiqul Gani, Ka Ming Ng





