Ammonia is synthesized from hydrogen and nitrogen. The synthesis gas is usually produced from hydrocarbons. The most
Question:
Ammonia is synthesized from hydrogen and nitrogen. The synthesis gas is usually produced from hydrocarbons. The most common raw materials are oil or natural gas, though coal and even peat can be used.
When produced from natural gas, the synthesis gas will be impure, containing up to 5% inerts, mainly methane and argon. The reaction equilibrium and rate are favored by high pressure. The conversion is low, about 15%, and so, after removal of the ammonia produced, the gas is recycled to the converter inlet. A typical process consists of a converter (reactor) operating at 350 bar, a refrigerated system to condense out the ammonia product from the recycle loop, and compressors to compress the feed and recycle gas. A purge is taken from the recycle loop to keep the inert concentration in the recycle gas at an acceptable level.
Using the following data, draw a flow diagram of the process and calculate the process stream flow rates and compositions for the production of 600 t/d ammonia.
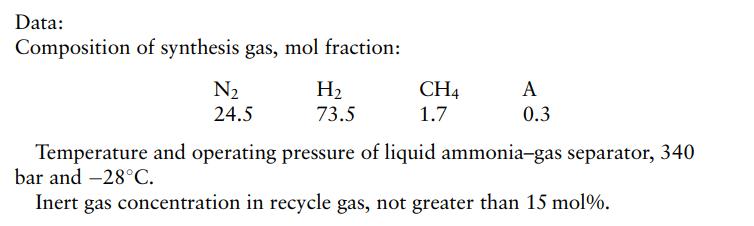
Step by Step Answer:






