Consider a binary equimolar mixture of acetone and chloroform at (300 mathrm{~K}) and (1 mathrm{~atm}). Because this
Question:
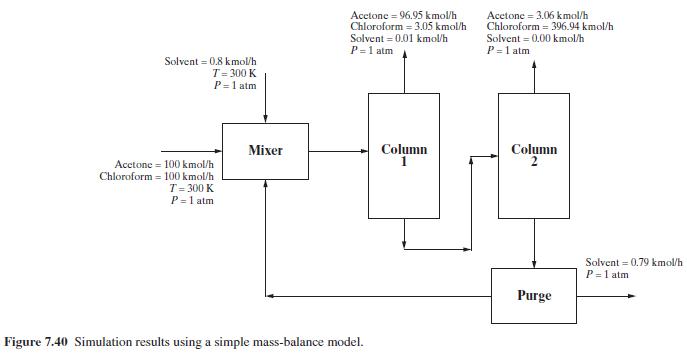
Consider a binary equimolar mixture of acetone and chloroform at \(300 \mathrm{~K}\) and \(1 \mathrm{~atm}\). Because this mixture forms a binary azeotrope, methyl- \(n\)-pentyl ether solvent is used to extract the chloroform from acetone. The flowsheet in Figure 7.40 for the process shows the results of solving a simple mass-balance model. Perform a rigorous simulation of the process by converting the two component-splitter modules into distillation-column modules. This involves finding appropriate specifications for the two distillation columns to yield approximately the mass balance given in the flowsheet. Assume a pressure of \(1 \mathrm{~atm}\) in all streams and estimate the temperatures of the other streams according to their phases (saturated liquid or vapor).
The separation in the mass-balance flowsheet gives an acetone product of approximately \(96 \%\) purity from column 1 and a chloroform product of approximately \(96 \%\) purity from column 2 . A makeup of \(0.8 \mathrm{kmol} / \mathrm{hr}\) of solvent is added to replace this amount lost from the purge stream. How can the product purity be improved and the solvent loss decreased?
Figure 7.40:-
Step by Step Answer:

Product And Process Design Principles Synthesis Analysis And Evaluation
ISBN: 9781119355243
4th Edition
Authors: Warren D. Seider, Daniel R. Lewin, J. D. Seader, Soemantri Widagdo, Rafiqul Gani, Ka Ming Ng





