Consider the hypothetical perfect separation of a mixture of ethylene and ethane into pure products by distillation
Question:
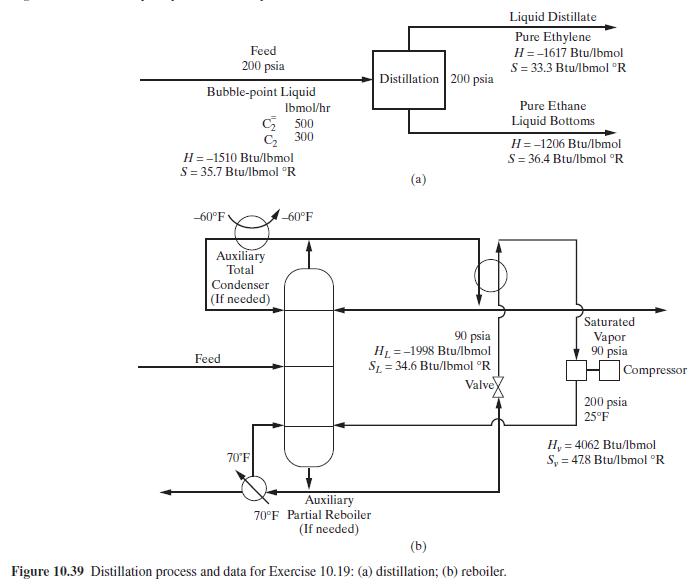
Consider the hypothetical perfect separation of a mixture of ethylene and ethane into pure products by distillation as shown in Figure 10.39.
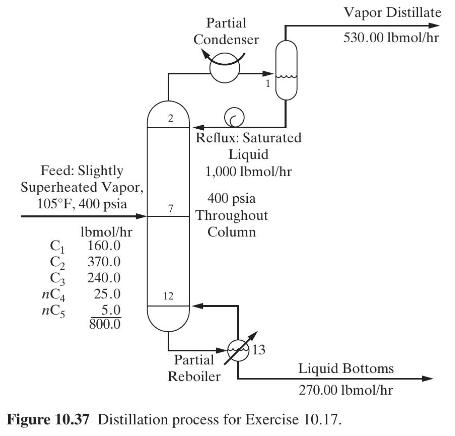
Two schemes are to be considered: conventional distillation and distillation using a heat pump with reboiler liquid flashing. In both cases, the column will operate at a pressure of \(200 \mathrm{psia}\), at which the average relative volatility is 1.55 . A reflux ratio of 1.10 times minimum, as computed from the Underwood equation, is to be used. Other conditions
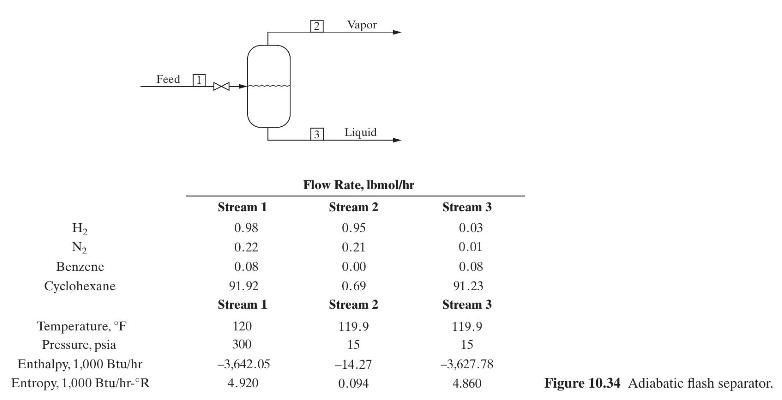
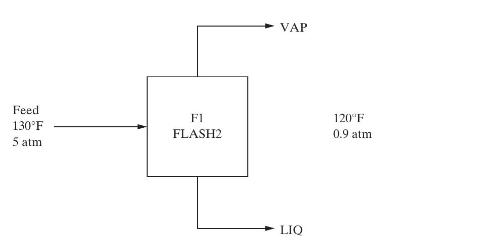
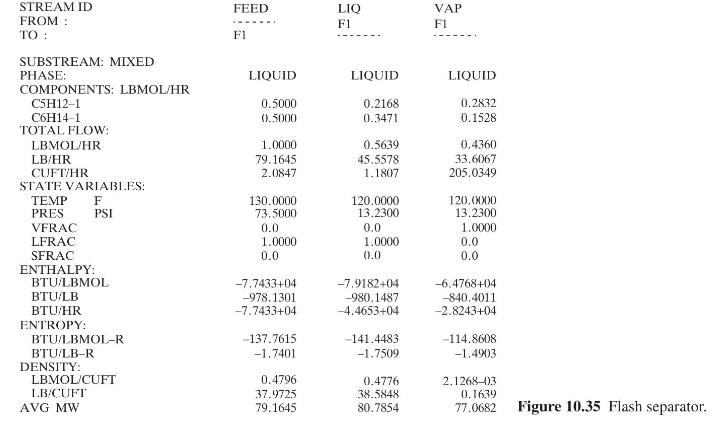
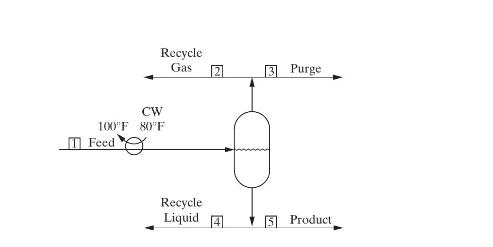
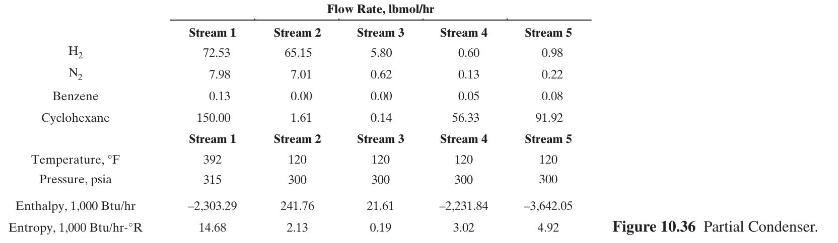
for the scheme using reboiler liquid flashing follow. Calculate the following for each scheme:
(a) Change in availability function \(\left(T_{0}=100^{\circ} \mathrm{F}\right)\)
(b) Lost work
(c) Thermodynamic efficiency Other thermodynamic data are:
Figure 10.39:-
Step by Step Answer:

Product And Process Design Principles Synthesis Analysis And Evaluation
ISBN: 9781119355243
4th Edition
Authors: Warren D. Seider, Daniel R. Lewin, J. D. Seader, Soemantri Widagdo, Rafiqul Gani, Ka Ming Ng





