In the manufacture of methyl ethyl ketone (MEK). The product MEK is extracted from a solution in
Question:
In the manufacture of methyl ethyl ketone (MEK). The product MEK is extracted from a solution in water using 1,1,2 trichloroethane as the solvent.
For a feed rate 2000 kg/h of solution, composition 30 per cent w/w MEK, determine the number of stages required to recover 95 per cent of the dissolved MEK; using 700 kg/h TCE, with counter-current flow.
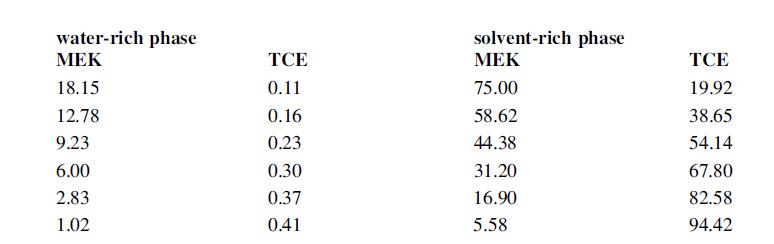
Tie-line data for the system MEK–water–TCE percentages w/w, from Newman et al. (1949).
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Engineering Design
ISBN: 9780081025994
6th Edition
Authors: Ray Sinnott, R.K. Sinnott, Sinnott Gavin Towler
Question Posted:





