(a) Calculate K at 25 C for the reaction Br 2 (g) 2 Br(g) from the...
Question:
(a) Calculate K at 25 °C for the reaction Br2(g) ⇌ 2 Br(g) from the thermodynamic data provided in Appendix 2A.
(b) What is the vapor pressure of liquid bromine?
(c) What is the partial pressure of Br(g) above the liquid in a bottle of bromine at 25 °C?
(d) A student wishes to add 0.0100 mol Br2 to a reaction and will do so by filling an evacuated flask with Br2 vapor from a reservoir that contains only bromine liquid in equilibrium with its vapor. The flask will be sealed and then transferred to the reaction vessel. What volume container should the student use to deliver 0.010 mol Br2(g) at 25°C?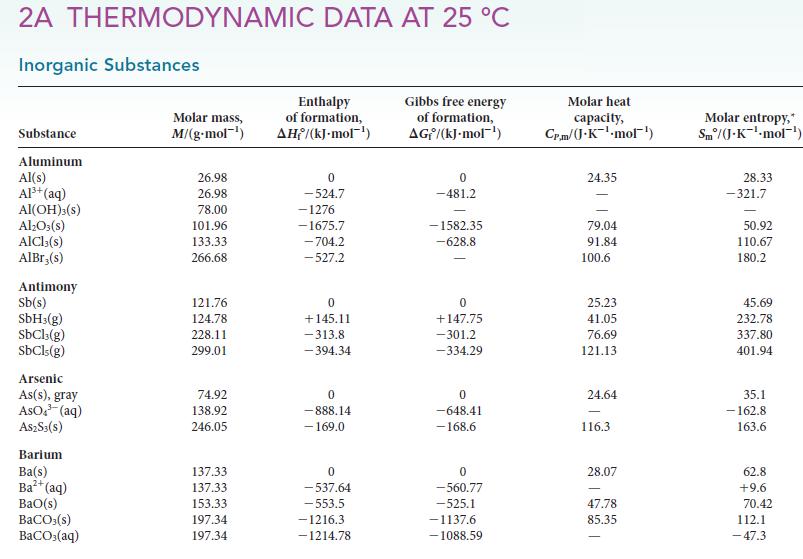
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





