(a) Calculate the work that must be done against the atmosphere for the expansion of the gaseous...
Question:
(a) Calculate the work that must be done against the atmosphere for the expansion of the gaseous products in the combustion of 1.00 mol C6H6(l) at 25 °C and 1.00 bar.
(b) Using data in Appendix 2A, calculate the standard enthalpy of the reaction.
(c) Calculate the change in internal energy, ΔU°, of the system.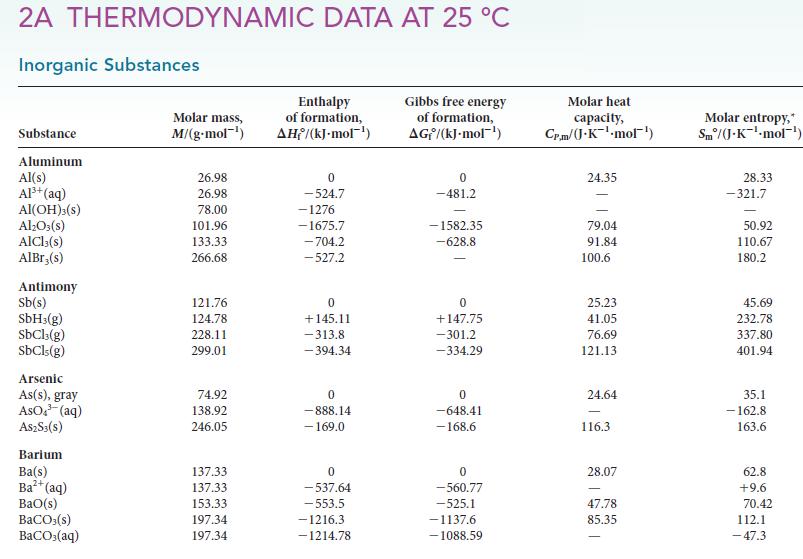
Transcribed Image Text:
2A THERMODYNAMIC DATA AT 25 °C Inorganic Substances Substance Aluminum Al(s) Al³+ (aq) Al(OH)3(S) Al₂O3(s) AlCl3(s) AlBr,(s) Antimony Sb(s) SbH3(g) SbCl3(g) SbCls (g) Arsenic As(s), gray AsO ³ (aq) A$2S3(S) Barium Ba(s) Ba²+ (aq) BaO(s) BaCO3(s) BaCO3(aq) Molar mass, M/(g.mol-¹) 26.98 26.98 78.00 101.96 133.33 266.68 121.76 124.78 228.11 299.01 74.92 138.92 246.05 137.33 137.33 153.33 197.34 197.34 Enthalpy of formation, AH/(kJ-mol-¹) 0 -524.7 -1276 -1675.7 -704.2 -527.2 0 +145.11 -313.8 -394.34 0 -888.14 - 169.0 0 -537.64 -553.5 -1216.3 -1214.78 Gibbs free energy of formation, AG/(kJ.mol-¹) 0 -481.2 -1582.35 -628.8 0 +147.75 -301.2 -334.29 0 -648.41 -168.6 0 -560.77 -525.1 -1137.6 -1088.59 Molar heat capacity, Cr.m/(J.K¹-mol¹) 24.35 79.04 91.84 100.6 25.23 41.05 76.69 121.13 24.64 116.3 28.07 47.78 85.35 Molar entropy, Sm/(J.K¹-mol-¹) 28.33 -321.7 50.92 110.67 180.2 45.69 232.78 337.80 401.94 35.1 -162.8 163.6 62.8 +9.6 70.42 112.1 -47.3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
a 372 ...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
(a) Calculate the work that must be done at 298.15 K against the atmosphere at 1.00 bar for the production of CO 2 (g) and H 2 O(g) in the combustion of 0.825 mol C 6 H 6 (l). (b) Calculate the...
-
Hyten Corporation On June 5, 1998, a meeting was held at Hyten Corporation, between Bill Knapp, Director of Marketing/Sales, and John Rich, director of engineering. The purpose of the meeting was to...
-
Calculate the work that must be done to charge a spherical shell of radius R to a total charge Q.
-
Upland Co.'s inventory records showed the following data accounted for in a perpetual inventory system. Date Units Unit Cost June 1 Inventory 1,250 $8.00 June 3 Purchases 2,500 8.40 June 7 Sales (at...
-
(a) For the three capacitor geometries in Problem 1.6 calculate the total electrostatic energy and express it alternatively in terms of the equal and opposite charges Q and Q placed on the conductors...
-
What is the value of having Gartner review its past predictions? As the world's leading research and advisory company on all matters relating to information technology, Gartner, Inc., attempts to see...
-
Research and prepare a report on the different approaches for accounting for carbon emissions under the EU ETS.
-
Criticize the following working paper that you are reviewing as senior auditor on the December 31 audit of Pratt Company. The client saved a copy of the deposit slip that is filled out. Per...
-
We have defined a PyTorch network class DAN for you. You need to implement the forward pass for your deep averaging network. To start, first implement average that averages the words in a review and...
-
Use the information in Table 4C.1 to calculate the changes in entropy of the surroundings and of the system for (a) The melting of 1.00 mol NH 3 (s) at its melting point; (b) The freezing of 1.00 mol...
-
Calculate the standard reaction entropy, enthalpy, and Gibbs free energy for each of the following reactions from data in Appendix 2A: (a) The decomposition of hydrogen peroxide: (b) The preparation...
-
The period of Mercury is approximately 88 days, and its orbit has eccentricity 0.205. How much longer does it take Mercury to travel from A to B than from B to A (Figure 2)?
-
How can organizations avoid, or at least minimize, unnecessary paperwork and processing?
-
Identify each of the following investments as either an economic investment or a financial investment. a. A company builds a new factory. b. A pension plan buys some Google stock. c. A mining company...
-
Describe three reasons why waiting is damaging to organizations?
-
How can overproduction be minimized?
-
How is transporting a waste? Provide an example to support your answer.
-
Suppose Coca-Cola and Pepsi announced plans to merge into a single global soft-drink company. What would be the possible effects on soft-drink consumers? What kind of regulatory scrutiny should the...
-
The area of square PQRS is 100 ft2, and A, B, C, and D are the midpoints of the sides. Find the area of square ABCD. B A
-
Given the following data: P4(s) + 6Cl(g) 4PC13(g) P4(s) + 50(g) P4010(S) PC13(g) + Cl(g) PCls(g) PC3(g) + O(g) ClPO(g) calculate AH for the reaction AH-1225.6 kJ AH = -2967.3 kJ = 84.2 kJ AH =...
-
Ammonia forms hydrogen-bonding intermolecular forces resulting in an unusually high boiling point for a substance with the small size of NH 3 . Can hydrazine (N 2 H 4 ) also form hydrogen-bonding...
-
Ammonia forms hydrogen-bonding intermolecular forces resulting in an unusually high boiling point for a substance with the small size of NH 3 . Can hydrazine (N 2 H 4 ) also form hydrogen-bonding...
-
Webber Ltd has 12 million common shares outstanding and long-term debt with a market value of $27 million. The Board of Directors has asked you to investigate the possibility of having a rights issue...
-
As the result of purchasing new equipment costing $580,000, ABC Inc. expects the number of units it produces and sales will increase next year by 11%. This past year the firm sold 85 units at a price...
-
Suppose that we want to have a yearly income of $50,000 in retirement. We expect to live 25 years in retirement and our inflation-adjusted rate of return on our safe investment is 3%. How much money...

Study smarter with the SolutionInn App


