A chemist needs to prepare a solution buffered at pH 4.30 using one of the following acids
Question:
A chemist needs to prepare a solution buffered at pH 4.30 using one of the following acids (and its sodium salt):
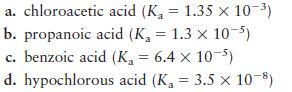
Calculate the ratio of [HA]/[A–] required for each system to yield a pH of 4.30. Which system will work best?
Transcribed Image Text:
a. chloroacetic acid (K = 1.35 x 10-) b. propanoic acid (K = 1.3 x 10-5) c. benzoic acid (K = 6.4 x 10-5) d. hypochlorous acid (K = 3.5 10-8)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
A pH of 430 corresponds to H 10430 antilog430 50 x 105 M Since K values rather tha...View the full answer

Answered By

Muhammad Ghyas Asif
It is my obligation to present efficient services to my clients by providing a work of quality, unique, competent and relevant. I hope you have confidence in me and assign me the order and i promise to follow all the instructions and keep time.
4.60+
109+ Reviews
203+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A student is asked to prepare a buffer solution at pH = 8.60, using one of the following weak acids: HA (Ka = 2.7 10-3), HB (Ka = 4.4 10-6), HC (Ka = 2.6 10-9). Which acid should she choose? Why?
-
A solution contains a weak monoprotic acid HA and its sodium salt NaA both at 0.1 M concentration. Show that [OH2] = Kw/Ka.
-
Which acid would you choose to combine with its sodium salt to make a solution buffered at pH 4.25? For the best choice, calculate the ratio of the conjugate base to the acid required to attain the...
-
At the beginning of compression in a diesel cycle, T = 540 R, P = 30 lbf/in. 2 , and the state after combustion (heat addition) is 2600 R and 1000 lbf/in. 2 . Find the compression ratio, the thermal...
-
Compose the expression for a quantity whose dimension is length, using velocity of light c, mass of a particle m, and Planck's constant h. What is that quantity?
-
What are the tax consequences to an S corporation and its shareholders if one of the requirements for a small business corporation is not met at some time in a tax year?
-
The stockholders' equity of Lakewood Occupational Therapy, Inc., on December 31, 2009, follows. On April 30, 2010, the market price of Lakewood's common stock was $14 per share and the company...
-
Assume Keiths employer reimburses him $4,200 for the move. Using the information from Problem 5, calculate Keiths moving expenses deduction using Form 3903 on Page 4-49.
-
1. Yesterday we discussed that F(t) = (r cos(c+t), -r sin(c+t)) and r(t) = (r sin(c+t), r cos(c+t)) both give the flow lines for the vector field F = (y,-x). I mentioned that the most general...
-
Hydrogen cyanide gas (HCN) is a powerful respiratory inhibitor that is highly toxic. It is a very weak acid (K a = 6.2 10 10 ) when dissolved in water. If a 50.0-mL sample of 0.100 M HCN is...
-
Calculate the change in pH that occurs when 0.010 mol of gaseous HCl is added to 1.0 L of each of the following solutions. Solution A: 5.00 M HC 2 H 3 O 2 and 5.00 M NaC 2 H 3 O 2 Solution B: 0.050 M...
-
By using more accurate approximations to Eq. (4.48a), explore the onset of the condensation near T = T c 0 . More specifically, do the following. (a) Approximate the numerator in Eq. (4.48a) by q 2 +...
-
What does this code do? while(1) { PTC->PDOR &= ~(OxOF < < 3); delayMs(5); }
-
Starting at t = 0 . 0 = 0 . 0 s , , suppose a ball has an initial velocity of 1 . 5 0 1 . 5 0 m / / s along the + + x- axis . . It also receives an acceleration of 2 . 8 0 2 . 8 0 m / / s along the +...
-
What will be the value inside the variables a and b after the given set of assignments? int a=12; int b-9; a=(a+b)/2; b=a++;
-
A final state in an NFA must not have outgoing arcs. Group of answer choices True False
-
If you form a language L 1 by reversing all the string s of a regular language L 2 , the L 1 is a regular language. Group of answer choices True False
-
Ebanks Company has a line of credit with United Bank. Ebanks can borrow up to $200,000 at any time over the course of the 2013 calendar year. The following table shows the prime rate expressed as an...
-
Sandcastles, Inc.s management has recently been looking at a proposal to purchase a new brick molding machine. With the new machine, the company would not have to buy bricks. The estimated useful...
-
Estimate the value of the quantum number n for the uppermost filled level in a one-dimensional line of copper atoms of length 1.0 mm.
-
What is the difference between homogeneous and heterogeneous alloys? Give examples of each type.
-
For which of the following molecules will dipoledipole interactions be important: (a) O 2 ; (b) O 3 ; (c) CO 2 ; (d) SO 2 ?
-
How do cognitive and emotional factors interact in shaping the dynamics of conflict, and what strategies can be employed to address both dimensions effectively ?
-
Theresa and Oliver, married filing jointly. Theresa is 5 9 years old; Oliver is 6 9 , and has deferred taking his social security of $ 4 , 2 0 0 monthly until he hits age 7 0 next year. He still...
-
Two perfectly transparent polymers are mixed. The resulting mixture is also perfectly transparent. What does it mean ?

Study smarter with the SolutionInn App


