(a) In an experiment, 5.0 mmol Cl 2 (g) was sealed into a reaction vessel of volume...
Question:
(a) In an experiment, 5.0 mmol Cl2(g) was sealed into a reaction vessel of volume 2.0 L and heated to 1200. K, and the dissociation equilibrium was established. What is the equilibrium composition of the mixture? Use the information in Table 5G.2.
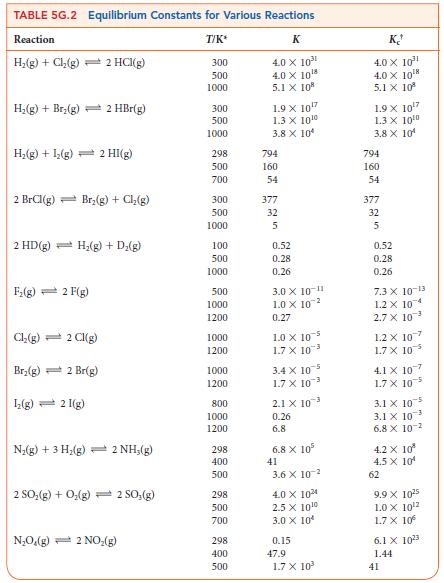
(b) If 5.0 mol Br2 is placed into the reaction vessel instead of the chlorine, what would be the equilibrium composition at 1200. K?
(c) Use your results from parts (a) and (b) to determine which is thermodynamically more stable relative to its atoms at 1200. K, Cl2, or Br2.
Transcribed Image Text:
TABLE 5G.2 Equilibrium Constants for Various Reactions Reaction T/K* H₂(g) + Cl₂(g) 2 HCl(g) H₂(g) + Brz(g) 2 HBr(g) H₂(g) + 1₂(g) 2 HI(g) 2 BrCl(g) Br₂(g) + Cl₂(g) 2 HD (g) H₂(g) + D₂(g) F₂(g) 2 F(g) Cl₂(g) = 2 Cl(g) Br₂(g) 2 Br(g) 1₂(g) 2 I(g) N₂(g) + 3 H₂(g) 2 NH₂(g) 2 SO₂(g) + O₂(g) - 2 SO₂(g) N₂O₂(g) 2 NO₂(g) 300 500 1000 300 500 1000 298 500 700 300 500 1000 100 500 1000 500 1000 1200 1000 1200 1000 1200 800 1000 1200 298 400 500 298 500 700 298 400 500 K 4.0 X 10³⁰ 4.0 X 10¹8 5.1 X 108 1.9 X 10¹7 1.3 X 10¹0 3.8 X 10 794 160 54 377 32 5 0.52 0.28 0.26 3.0 X 10-¹1 1.0 X 102 0.27 1.0 X 105 1.7 X 10³ 3.4 X 105 1.7 X 10³ 2.1 X 10 0.26 6.8 6.8 X 10² 41 3.6 X 102 4.0 X 10²4 2.5 X 10¹0 3.0 X 10 0.15 47.9 1.7 X 10³ K 4.0 X 10³¹¹ 4.0 X 10¹ 5.1 X 10² 1.9 x 10¹7 1.3 X 10⁰0 3.8 X 10 794 160 54 377 32 5 0.52 0.28 0.26 7.3 X 10-13 1.2 X 104 2.7 X 10³ 1.2 X 107 1.7 X 105 4.1 x 107 1.7 X 10 3.1 x 10-5 3.1 X 103 6.8 X 10² 4.2 X 10² 4.5 X 10¹ 62 9.9 X 10²5 1.0 X 10¹2 1.7 X 106 6.1 x 1023 1.44 41
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
Equilibrium Composition of Chlorine and Bromine Dissociation Table 5G2 provides the equilibrium constants K for the dissociation of various diatomic m...View the full answer

Answered By

ANDREW KIPRUTO
Academic Writing Expert
I have over 7 years of research and application experience. I am trained and licensed to provide expertise in IT information, computer sciences related topics and other units like chemistry, Business, law, biology, biochemistry, and genetics. I'm a network and IT admin with +8 years of experience in all kind of environments.
I can help you in the following areas:
Networking
- Ethernet, Wireless Airmax and 802.11, fiber networks on GPON/GEPON and WDM
- Protocols and IP Services: VLANs, LACP, ACLs, VPNs, OSPF, BGP, RADIUS, PPPoE, DNS, Proxies, SNMP
- Vendors: MikroTik, Ubiquiti, Cisco, Juniper, HP, Dell, DrayTek, SMC, Zyxel, Furukawa Electric, and many more
- Monitoring Systems: PRTG, Zabbix, Whatsup Gold, TheDude, RRDtoo
Always available for new projects! Contact me for any inquiries
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
(a) In an experiment, 2.0 mmol Cl 2 (g)was sealed into a reaction vessel of volume 2.0 L and heated to 1000. K to study itsdissociation into Cl atoms. Use the information in Table 5G.2 to calculate...
-
Phosphorus pentachloride, PCl 5 , is used to convert alcohols (such as CH 3 CH 2 OH) to alkyl chlorides (such as CH 3 CH 2 Cl). If you were an industrial chemist, you might be asked to prepare some...
-
Dinitrogen oxide, N 2 O, colloquially called laughing gas, was first used as an anesthetic in dentistry in 1844. Suppose that you are a chemist attempting to prepare N 2 O from N 2 and O 2 ; you...
-
Solve the inequality. Write the solution in interval notation. |-3x + 1 5
-
A polyisoprene membrane of 0.8-pm thickness is to be used to separate a mixture of methane and H2. Using the data in able 14.9 and the following compositions, estimate the mass transfer flux of each...
-
Assume that a production line operates such that the production lot size model of Section 14.2 is applicable. Given D = 6400 units per year, Co = $100 and Ch = $2 per unit per year, compute the...
-
Check whether the following can define probability distributions and explain your answers. (a) \(f(x)=\frac{1}{4}\) for \(x=10,11,12,13\) (b) \(f(x)=\frac{2 x}{5}\) for \(x=0,1,2,3,4,5\) (c)...
-
Sommer Graphics Company was organized on January 1, 2019, by Krystal Sommer. At the end of the first 6 months of operations, the trial balance contained the accounts shown below. Analysis reveals the...
-
Trump Ltd. decided to increase its bill payment time from 20 days to 40 days. It was said that they wanted to exercise some cost control and optimize cash flow. i) What impact will this have on...
-
Pure Plant Beauty is a sole proprietorship that has a developed a new line of skin care and makeup that uses natural and organic ingredients. The company showed the following adjusted account...
-
An item is marked down by the same percentage as the rate of markup on selling price. Will the reduced operating profit be positive, negative, or zero? Explain.
-
The following groups are found in some organic molecules. Which are hydrophilic and which are hydrophobic: (a) OH; (b) CH 2 CH 3 ; (c) CONH 2 ; (d) Cl?
-
Silicon Dynamics has developed a new computer chip that will enable it to begin producing and marketing a personal computer if it so desires. Alternatively, it can sell the rights to the computer...
-
Juan borrows $25,000 at 7 percent compounded annually. If the loan is repaid in five equal annual payments, what will be the size of Juans payments if the first payment is made 1 year after borrowing...
-
You decide to place $12,000 on deposit for 4 years. The bank offers you 6 percent compounded annually. a. What is the total amount of money in the account at the end of 4 years? b. What value of...
-
You purchase a quarter section (160 acres) of land for \($176\),000 today and sell it in exactly 9 years for \($525\),000 at auction. At what annual compound rate did the value of your land grow?
-
If Kathy borrows $11,000 to remodel her beach house at 7 percent compounded annually for 5 years, what is the principal, the interest, and the final amount paid if all are paid at the end of year 5?
-
Adriana wishes to accumulate $2,000,000 in 35 years. If 35 end-of-year deposits are made into an account that pays interest at a rate of 7 percent compounded annually, what size deposit is required...
-
Taylor dies on February 19 of the current year. Among the assets in his estate are 500 shares of Dane Company preferred stock. Taylor paid $14 per share for the stock on August 13, 2001. Market...
-
Use translations to graph f. f(x) = x-/2 +1
-
Propose a synthesis for each of the following transformations. a. b. c. d. Br
-
Propose a plausible synthesis for each of the following transformations. a. b. c. d. e. f.
-
Show at least two different methods for preparing 1-methylcyclohexene from 1-methylcyclohexanol.
-
Suppose there is a reduced supply of canned tomatoes from China to Australia during COVID-19. Also, consumers are increasingly feeling concerned about supply shortages. What will be the impact of...
-
The Exchange Rate Volatility (ERV) and Trade Balance (TB) relationship by using monthly data over the period January 2010 to December 2019 and a nonlinear regression model, namely the Nonlinear...
-
What is Cullumber's times interest earned for 2025? Cullumber Company's 2025 financial statements contain the following selected data: Income taxes $44000 Interest expense 24000 Net income 56800

Study smarter with the SolutionInn App


