(a) What is the overall reaction for the following mechanism? (b) Write the rate law based on...
Question:
(a) What is the overall reaction for the following mechanism?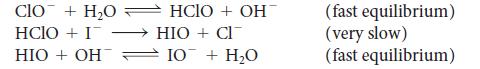
(b) Write the rate law based on this mechanism.
(c) How will the reaction rate depend on the pH of the solution?
(d) How would the rate law differ if the reactions were carried out in an organic solvent?
Transcribed Image Text:
CIO + H₂O HCIO + I HIO + OH HCIO+ OHT HIO + CI 10 + H₂O (fast equilibrium) (very slow) (fast equilibrium)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
a overall reaction ClO I IO kk CIOI k OH b rate c As the pH incre...View the full answer

Answered By

Lina Anil
I am Lina Anil have 8 years of teaching experience in biochemistry and microbiology.Guided UG and PG students for their academic projects.Co ordinated various workshops and student awareness programs.I have thorough knowledge in biochemistry related subjects. I am also interested in programming languages like CPP and python.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
The production of maleic anhydride by the air oxidation of benzene was studied using a vanadium pentoxide catalyst [Chem Eng Sci, 43, 1051 (1988)]. The reactions that occur are C6H6 + 9/ 2 O2 C4H2O3...
-
A reaction A + B -- C obeys the following rate law: Rate = k[B]2. (a) If [A] is doubled, how will the rate change? Will the rate constant change? Explain. (b) What are the reaction orders for A and...
-
A proposed two-step mechanism for the destruction of ozone in the upper atmosphere is a. What is the overall balanced equation for the ozone destruction reaction? b. Which species is a catalyst? c....
-
A manufacturing company reports the following information for the month of May. Note: Assume all raw materials were used as direct materials. Activities for May Advertising expense Raw materials...
-
Look at Table 9.2. What would the four countries? betas be if the correlation coefficient for each was 0.5? Do the calculation and explain. Ratio of Standard Correlation Betat Coefficient Deviations*...
-
Ling Corporation uses the equity method to account for its ownership of 35% of the common stock of Gorman Packing. During 2014, Gorman reported a net income of $80,000 and declares and pays cash...
-
In addition to the size variables, we also have information on several binary variables. The variable URBAN is used to indicate the facility's location. It is one if the facility is located in an...
-
Member AB is supported at B by a cable and at A by a smooth fixed square rod which fits loosely through the square hole of the collar. If F = {20i ? 40j ? 75k}, determine the x, y, z components of...
-
A $1,000 par value bond has a 12% coupon rate (paid annually). It has 10 years remaining to maturity. If bonds of similar risk and maturity currently yield 8%, what should this bond's price be?
-
The pre-equilibrium and the steady-state approximations are two different approaches to deriving a rate law from a proposed mechanism. For the following mechanism, determine the rate law (a) By the...
-
Write the formulas for the oxoanions of the following elements in which the element is found with its highest oxidation number (see Fig. 9A.7). In each case, the charge of the oxoanion is given in...
-
The following information was taken from the balance sheet of Laribee Company (amounts are in thousands of dollars): Current liabilities* ............................ $ 24,480 Long-term debt...
-
Prepare an Income Statement for the year ending 31 December 2022. Prepare a Balance Sheet as at 31 December 2022. The balance sheet of Torrent and Co at the end of the first of trading is as...
-
a. The traveling salesman problem (TSP) is to find the shortest tour through a given set of n cities that visits each city exactly once before returning to the city where it started. Solve the...
-
John MacLean plans to begin his consulting business as a proprietary company (John Maclean Pty Ltd) on 1 January 2022. He has provided projected data for the first 36 months of operations. John...
-
Find the z-transform for each of the following sequences: (a) 4u(n-4) (b) 2(-0.8)"u(n) (c) 4e-2nu(n) (d) 4(0.8)" cos(0.1n)u(n) (e) e-2(n-3) sin(0.27(n 3))u(n 3)
-
1. It is easy to show that in fcc lattice four basal atoms: (000),(0, , ), (,0, ), (, ,0) form regular tetrahedron with the center at ( , ). It is called the tetrahedral void. The lattice cell...
-
What are the four steps in decision making?
-
How does the organizational structure of an MNC influence its strategy implementation?
-
Evaluate the average of the square of the linear momentum of the quantum harmonic oscillator, p x 2 for the ground state (n = 0) and first two excited states (n = 1and n = 2).
-
Show by carrying out the appropriate integration that the total energy eigenfunctions for the harmonic oscillator 0 (x) ( /) 1/4 e -(1/2)ax2 and 2 (x) ( /4) 1/4 (2x - 1) e -(1/2)ax2 are orthogonal...
-
Two 3.25-g masses are attached by a spring with a force constant of k = 450. kg s 2 . Calculate the zero point energy of the system and compare it with the thermal energy k B T at 298 K. If the zero...
-
Steve has been operating Castle Creek Restaurant in Ontario for the past several years. On the basis of the information that Steve's accountant filed with the CRA during the prior year, Castle Creek...
-
Rhonda Brennan found her first job after graduating from college through the classifieds of the Miami Herald. She was delighted when the offer came through at $ 1 6 . 1 0 ?per hour. She completed her...
-
Let w = F(x, y), where x=u 3uv-v and y = g(u, v), F and g are differentiable, and g(5, 1) =0, gu (5, 1) = 29, 9, (5, 1) = 6, F(9, 0) 3, F(9, 0) = 14, Fy(9, 0) = 5. Iw Find when u 5 and v = 1. Type...

Study smarter with the SolutionInn App


