Question:
Although molar solubilities are often of interest, you might find it difficult to locate the appropriate data. Solubility constants, however, are often easier to find, and can be converted to molar solubilities. According to Table 6I.1, Ksp = 5.0 * 10–6 for chromium(III) iodate in water at 25°C. Estimate the molar solubility of the compound at 25°C.
PLAN Set up the solubility product in terms of the molar solubility, taking into account the stoichiometric relations implied by the chemical equation for the equilibrium, and then solve for the molar solubility.
What should you assume? Assume that the salt dissociates completely in water and that the anion is not protonated by water.
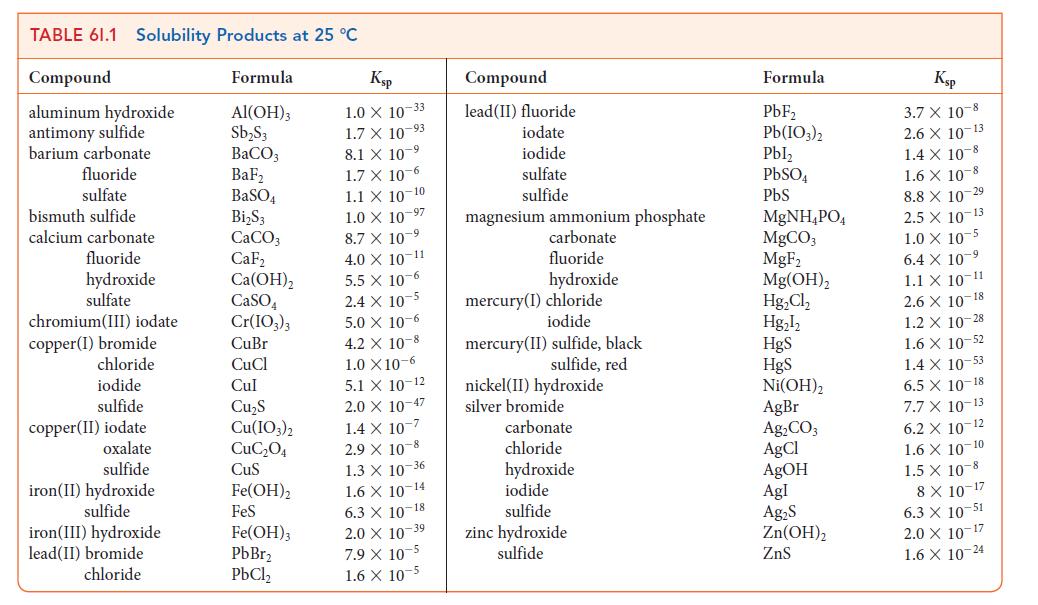
Transcribed Image Text:
TABLE 61.1 Solubility Products at 25 °C
Compound
aluminum hydroxide
antimony sulfide
barium carbonate
fluoride
sulfate
bismuth sulfide
calcium carbonate
fluoride
hydroxide
sulfate
chromium(III) iodate
copper (1) bromide
chloride
iodide
sulfide
copper(II) iodate
oxalate
sulfide
iron (II) hydroxide
sulfide
iron(III) hydroxide
lead(II) bromide
chloride
Formula
Al(OH)3
Sb₂S3
BaCO3
BaF₂
BaSO4
Bi₂S3
CaCO3
CaF₂
Ca(OH) ₂
CaSO4
Cr(103) 3
CuBr
CuCl
Cul
Cu₂S
Cu(IO3)2
CuC₂04
CuS
Fe(OH)2
FeS
Fe(OH)3
PbBr₂
PbCl₂
Ksp
1.0 X 10-33
1.7 X 10-93
8.1 X 10-9
1.7 x 10-6
1.1 X 10-10
1.0 X 10-97
8.7 X 10-9
4.0 X 10-11
5.5 x 10-6
2.4 x 10-5
5.0 x 10-6
4.2 X 10-8
1.0 X10-6
5.1 X 10-12
2.0 X 10-47
1.4 X 107
2.9 X 10 8
1.3 X 10-36
1.6 X 10-14
6.3 X 10-18
2.0 X 10-39
7.9 X 10-5
1.6 X 10-5
Compound
lead(II) fluoride
iodate
iodide
sulfate
sulfide
magnesium ammonium phosphate
carbonate
fluoride
hydroxide
mercury(I) chloride
iodide
mercury (II) sulfide, black
sulfide, red
nickel(II) hydroxide
silver bromide
carbonate
chloride
hydroxide
iodide
sulfide
zinc hydroxide
sulfide
Formula
PbF₂
Pb(103)2
Pbl₂
PbSO4
PbS
MgNH₂PO4
MgCO3
MgF₂
Mg(OH)₂
Hg₂Cl₂
Hg₂l₂
HgS
HgS
Ni(OH)2
AgBr
Ag₂CO3
AgCl
AgOH
AgI
Ag₂S
Zn(OH)₂
ZnS
Ksp
3.7 X 10 8
2.6 X 10-13
1.4 X 10-8
1.6 X 10-8
8.8 X 10-29
2.5 X 10-13
1.0 X 105
6.4 X 10-9
1.1 X 10-11
2.6 X 10-18
1.2 X 10-28
1.6 X 10-52
1.4 x 10-53
6.5 X 10-18
7.7 X 10-13
6.2 X 10-12
1.6 X 10-10
1.5 X 10 8
8 X 10-17
6.3 X 10-51
2.0 X 10-17
1.6 X 10-24







