An aqueous solution of Na 2 SO 4 was electrolyzed for 30.0 min; 25.0 mL of oxygen
Question:
An aqueous solution of Na2SO4 was electrolyzed for 30.0 min; 25.0 mL of oxygen was collected at the anode over water at 22°C and a total pressure of 722 Torr. Determine the current that was used to produce the gas. See Table 5A.2 for the vapor pressure of water.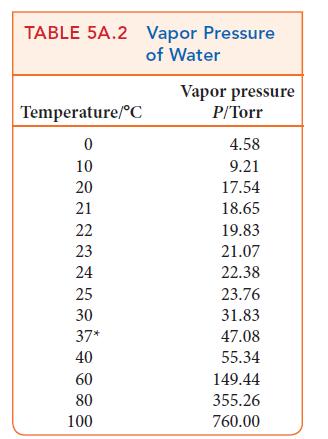
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





