Calculate the (mathrm{pH}) of the cathode compartment for the following reaction given (mathscr{E}_{text {cell }}=3.01 mathrm{~V}) when
Question:
Calculate the \(\mathrm{pH}\) of the cathode compartment for the following reaction given \(\mathscr{E}_{\text {cell }}=3.01 \mathrm{~V}\) when \(\left[\mathrm{Cr}^{3+}ight]=\) \(0.15 \mathrm{M},\left[\mathrm{Al}^{3+}ight]=0.30 \mathrm{M}\), and \(\left[\mathrm{Cr}_{2} \mathrm{O}_{7}{ }^{2-}ight]=0.55 \mathrm{M}\).
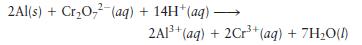
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





