Calculate the standard Gibbs free energy of each of the following reactions: (a) I(g) 2 I(g), K
Question:
Calculate the standard Gibbs free energy of each of the following reactions: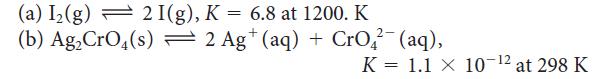
Transcribed Image Text:
(a) I₂(g) 2 I(g), K = 6.8 at 1200. K (b) Ag₂ CrO4(s) = 2 Ag+ (aq) + CrO₂²(aq), K 1.1 X 10-12 at 298 K
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
a AG ...View the full answer

Answered By

Ishrat Khan
Previously, I have worked as an accounting scholar at acemyhomework, and have been tutoring busines students in various subjects, mostly accounting. More specifically I'm very knowledgeable in accounting subjects for college and university level. I have done master in commerce specialising in accounting and finance as well as other business subjects.
5.00+
134+ Reviews
427+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
The oxidation of SO 2 to SO 3 is one of the reactions involved in the formation of acid rain. If you want to predict the spontaneous direction of the reaction for a specific mixture of the gases, you...
-
(a) Calculate the standard Gibbs free energies of formation of the halogen atoms X (g) at 1000. K from data available in Table 5G.2. (b) Show how these data correlate with the XX bond strength by...
-
Ammonia survives indefinitely in air. An agronomist studying how long ammonia survives in soil might need to know whether it survives because its oxidation is not spontaneous under ordinary...
-
In Exercises 3538, evaluate C F dr. F(x, y, z) = xi + yj + zk C: r(t) = 2 cos ti + 2 sin tj + tk, 0t 2
-
Find the current in each resistor of the circuit shown in Figure. 12 V
-
The workweek for all employees is 40 hours, except for the students 36-hour schedule. The office is open from 8:00 a.m. to 5:00 p.m. each day, except weekends. Glo-Brite allows office employees one...
-
Consider the data from Example 7.9. In Section 7.7.4 Computation Lab: Nearest Neighbour Propensity Score Matching, marital1982 was included when fitting the propensity score model propmod_nhefs....
-
Few IPOs have garnered as much attention as social media giant Facebooks public offering on May 18, 2012. It was the biggest IPO in Internet history, easily topping Googles initial public offering...
-
Assume the following: Sales price and cost of a property are $ 3 , 0 0 0 , 0 0 0 and $ 2 , 1 0 0 , 0 0 0 , respectively, so that the total profit to be recognized is $ 9 0 0 , 0 0 0 . Seller received...
-
Use functional decomposition to find the best implementation of the function f (x 1 , . . . , x 5 ) = m(1, 2, 7, 9, 10, 18, 19, 25, 31) + D(0, 15, 20, 26). How does your implementation compare with...
-
The equilibrium constant Kc for the reaction N 2 (g) + O 2 (g) 2 NO (g) at 1200C is 1.00 * 10 5 . Calculate the equilibrium molar concentrations of NO, N 2 , and O 2 in a reaction vessel of volume...
-
Distinguish between an emulsion and a gel. Give at least one example of each.
-
Perform the indicated operation, and write each answer in lowest terms.
-
There are two events \(A\) and \(B . P(A)=7\) and \(P(B)=8 . P\left(\begin{array}{ll}A & B\end{array} ight)=1\). (a) Are \(A\) and \(B\) independent events? Explain why or why not. (b) Find...
-
Let \(Y\) be normally distributed with mean \(=120\) and variance \({ }^{2}=64\). (a) Find \(P\left(\begin{array}{ll}Y & 130\end{array} ight)\). (b) Find \(P\left(\begin{array}{ll}Y & 135\end{array}...
-
There are two events \(A\) and \(B . P(A)=4\) and \(P(B)=4 . P\left(\begin{array}{ll}A & B\end{array} ight)=\) 24 . (a) Are \(A\) and \(B\) independent events? Explain why or why not. (b) Find...
-
Derive an expression for the law of corresponding states for a gas represented by the following expression: \[p=\frac{\Re T}{v-b}-\frac{a}{T v^{2}}\] \[\left[p_{\mathrm{R}}=\frac{8 T_{\mathrm{R}}}{3...
-
Let \(Z\) have the standard normal distribution. (a) Find \(P\left(\begin{array}{lll}0 & Z & 152\end{array} ight)\). (b) Find \(P\left(\begin{array}{ll}Z & 211\end{array} ight)\). (c) Find...
-
Determine the payback period without interest for Problem 5. If the maximum allowable payback period without interest for your company is three years, should your company purchase the dump truck? In...
-
Read Case Study Google: Dont Be Evil Unless and answer the following: Why do you think Google was adamant about not wanting to supply information requested by the government concerning the Child...
-
When (R)-3-bromo-2, 3-dimethylpentane is treated with sodium hydroxide, four different alkenes are formed. Draw all four products, and rank them in terms of stability. Which product do you expect to...
-
When 3-bromo-2, 4-dimethylpentane is treated with sodium hydroxide, only one alkene is formed. Draw the product and explain why this reaction has only one regiochemical outcome.
-
Predict the major product for each of the following E2 reactions: a. b. c. d. NaOH Br NaOH Br
-
How do emerging technologies such as biofeedback systems and virtual reality environments facilitate the implementation of personalized stress reduction interventions, and what are the potential...
-
Presented below is information related to Sunbuck's Coffee Club for its fiscal year ending April 30, 2023. During the year, the Board of Directors decided to discontinue the operations of its entire...
-
Constructing Balance Sheets and Determining Income Following is balance sheet information for Lynch Services at the end of Year 2 (the most recent year) and Year 1. December 31,Year 2 December...

Study smarter with the SolutionInn App


