Calculate the vapor pressure of the solvent in each of the following solutions. Use Table 5A.2 to
Question:
Calculate the vapor pressure of the solvent in each of the following solutions. Use Table 5A.2 to find the vapor pressure of water in
(a) An aqueous solution at 100 °C in which the mole fraction of sucrose is 0.100;
(b) An aqueous solution at 100 °C in which the concentration of sucrose is 0.100 mol · L–1.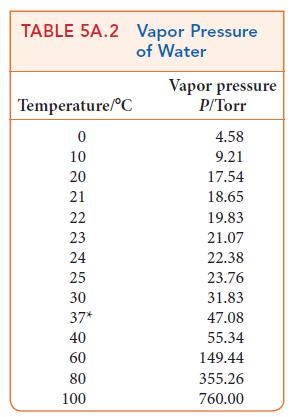
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





