Consider a solution made by mixing (500.0 mathrm{~mL}) of 4.0 (M mathrm{NH}_{3}) and (500.0 mathrm{~mL}) of (0.40
Question:
Consider a solution made by mixing \(500.0 \mathrm{~mL}\) of 4.0 \(M \mathrm{NH}_{3}\) and \(500.0 \mathrm{~mL}\) of \(0.40 \mathrm{M} \mathrm{AgNO}_{3} . \mathrm{Ag}^{+}\)reacts with \(\mathrm{NH}_{3}\) to form \(\mathrm{AgNH}_{3}{ }^{+}\)and \(\mathrm{Ag}\left(\mathrm{NH}_{3}ight)_{2}{ }^{+}\):
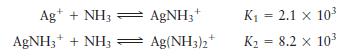
Determine the concentration of all species in solution.
Transcribed Image Text:
Ag+ + NH3 AgNH3 + AgNH3+ + NH3 = Ag(NH3)2+ K = 2.1 x 10 K = 8.2 x 10
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
The reaction between silver ions Ag and ammonia NH3 is a Lewis acidbase reaction Silver ion acts as ...View the full answer

Answered By

Ajeet Singh
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions.
I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life.
I have also done one-to-one tutoring with 100+ students and help them achieve great subject knowledge.
I have expertise in computer subjects like C++, C, Java, and Python programming and other computer Science related fields.
Many of my student's parents message me that your lessons improved their children's grades and this is the best only thing you want as a teacher. I have done plenty of studying and lots of learning on many exciting and challenging topics. All these experiences have influenced my decision to take on the teaching role in various capacities. As a tutor, I am looking forward to getting to understand your needs and helping you achieve your academic goals. I'm highly flexible and contactable. I am available to work on short notice since I only prefer to work with very small and select groups of students.
4.90+
7+ Reviews
15+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
If P = 20 lb, replace the three couples with a single equivalent couple, specifying its magnitude and the direction of its axis. 15 in. 16 lb 15 in, 40 1b 16 lb 40 lb -P 10 in 10 in 10 in
-
(b) A manufacturer of a certain electronic tube claims that the average life span of tubes will exceed 1000 hours from the past experience the standard deviation is known to be 120 hours. A retailer...
-
The 23-in. vertical rod CD is welded to the midpoint C of the 50-in. rod AB. Determine the moment about AB of the 235-lb force P. 32 in 24 in 17 inC GT 30 in 12 16 in S in 21 in
-
The ultimate test of fluency in MS and IR is whether you can determine a moderately complex structure from just the MS and the IR, with no additional information. The IR and MS of a compound are...
-
Show that the energy of a particle (charge e) in a synchrotron, in the relativistic limit (v c), is given by E (in eV) = Brc, where B is magnetic field strength and r the radius of the orbit (SI...
-
Figure 8.28 shows a switch in a virtual-circuit network. Find the output port and the output VCI for packets with the following input port and input VCI addresses: a. Packet 1: 3, 78 b. Packet 2: 2,...
-
What are the different types of consulting and litigation support activities for fraud and forensic accounting professionals?
-
This information relates to Crofoot Real Estate Agency. Oct 1 Stockholders invest $30,000 in exchange for common stock of the corporation. 2. Hires an administrative assistant at an annual salary of...
-
Explain the advantages of the relational model. Conduct some light research on the Web and describe how it differs from other data models. Explain some alternatives to the relational model. Please...
-
Solutions of sodium thiosulfate are used to dissolve unexposed \(\mathrm{AgBr}\) in the developing process for black-andwhite film. What mass of \(\mathrm{AgBr}\) can dissolve in \(1.00 \mathrm{~L}\)...
-
A solution is prepared by adding \(0.090 \mathrm{~mol}\) of \(\mathrm{K}_{3}\left[\mathrm{Fe}(\mathrm{CN})_{6}ight]\) to \(0.60 \mathrm{~L}\) of \(2.0 \mathrm{M} \mathrm{NaCN}\). Assuming no volume...
-
On the graph below point B is symmetric to point A about the y-axis and point C is symmetric to point B across the x-axis. What is the area of triangle ABC? A. 2 B. 4 C. 8 D. 16 E. 24 B A = (2,4)
-
Iramba Sea Foods Investment is negotiating a Loan from Tanzania Investments Bank which has kindly requested the Company to prepare and submit their cash budget for the first half year. The following...
-
Dilemmas As a purchasing manager, you may be faced with making product decisions that conflict with your own strongly-held values and beliefs. For example: You are concerned about the environment,...
-
What is the relationship between communication and health inequality? Specify the pathways through which communication influences health inequality.
-
2. The Quickie Mart receives a shipment of batteries on Jan 20th. 15 cases are delivered. The price is $45 per case with discounts of 6% and 5% and payment terms of 1/15, n/30. a. What is the last...
-
Sarah Green has been an accountant for 12 years. She worked in a small medical practice for two years after earning her BS in Accounting and then chose to work freelance for five years. For the past...
-
Considering the international locales in this case, why was Lindholm able to bring an action against Brant in Connecticut?
-
The senior management at Davis Watercraft would like to determine if it is possible to improve firm profitability by changing their existing product mix. Currently, the product mix is determined by...
-
Each of the pictures below shows a molecular view of a system undergoing a change. In each case, indicate whether heat is absorbed or given off by the system and whether expansion work is done on or...
-
Calculate the change in entropy when the pressure of 5.75 g of helium gas is decreased from 320.0 kPa to 40.0 kPa while the temperature decreases from 423 K to 273 K. Assume ideal behavior.
-
Use data in Table 4H.1 or Appendix 2A to calculate the standard entropy change for each of the following reactions at 25C. For each reaction, interpret the sign and magnitude of the reaction entropy....
-
Luis, a student from Malta, had $7,500 in wages reported to him on Form W-2. Although all of his wages are excluded from tax by treaty, he is required to file a tax return. a. True b. False unanswered
-
At the end of the year a corporation may incur income tax expense, this transaction is recorded with? Select one: a. None of the available choices b. Increase to expense account c. Increase to a...
-
Do you and your partner fight fair so that you can disagree without undermining your relationship? To what extent do you feel that sexual satisfaction and relationship satisfaction go together? If...

Study smarter with the SolutionInn App


