Consider atoms with the following electron configurations: Which atom has the largest first ionization energy, and which
Question:
Consider atoms with the following electron configurations:
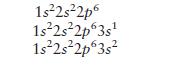
Which atom has the largest first ionization energy, and which has the smallest second ionization energy? Explain your choices.
Transcribed Image Text:
1s2s2p6 1s2s2p63s 1s2s2p 3s
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
The atom with the largest value of I is the one with the configu ration 1s2...View the full answer

Answered By

Elias Gichuru
am devoted to my work and dedicated in helping my clients accomplish their goals and objectives,providing the best for all tasks assigned to me as a freelancer,providing high quality work that yields high scores.promise to serve them earnestly and help them achieve their goals.i have the needed expertise,knowledge and experience to handle their tasks.
4.80+
325+ Reviews
859+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Graph f(x) - x, g(x) x +3 and (x) x5. Calculate the derivatives of f, g and h.
-
Identify the outer electron configurations for the (a) alkali metals, (b) alkaline earth metals, (c) halogens, (d) noble gases. For each electronic configuration given, choose the electronic...
-
Consider the formation of atomic hydrogen in the reaction c + H+ = H, where e is an electron, as the adsorption of an electron on a proton H+. (a) Show that the equilibrium concentrations of the...
-
Consider a situation with J identical firms that have marginal abatement cost functions for j=1,,J. The marginal damage function is equal to D'(E)=d.EDetermine the optimal allocation and the optimal...
-
Where g is the gravitational field strength, determine the value of T. T = 27V V/gA
-
What mechanisms are used to address the incentive conflicts in corporations?
-
Martial Arts Schools, Inc., is authorized to issue 500,000 shares of \$1 par common stock. The company issued 80,000 shares at \(\$ 4\) per share. When the market price of common stock was \(\$ 6\)...
-
Donald Barker, a wealthy Oregon resident, went to the law firm Winokur, Schoenberg, Maier, Hamerman & Knudson to have his estate planned. An attorney at the firm repeatedly told Barker that he could...
-
Why are Dermotherm and La Fantaine running these promotions? Are they achieving their objectives? Should Benati react? Why or why not? If so, how should Benati react? What are Benati's alternatives?...
-
Predict the trend in radius of the following ions: Be 2+ , Mg 2+ , Ca 2+ , and Sr 2+ .
-
The first ionization energy for phosphorus is 1060 kJ/mol, and that for sulfur is 1005 kJ/mol. Why?
-
Rupert bought a house in Manchester on 1 November 2001 for 75,000. He occupied the house as his principal private residence until 1 November 2005 when he left to work abroad for a year, living in...
-
Consider the following information: 1 - Jan - 2 0 3 1 - Dec - 2 0 Inventories: Direct Materials $ 1 1 , 0 0 0 $ 8 , 0 0 0 Work in Process 1 3 , 0 0 0 1 2 , 0 0 0 Finished Goods 9 , 5 0 0 6 , 5 0 0...
-
An analysis of monthly sales compared with past years and budgets is a form of what type of testing?
-
How do cultural paradigms and sociopolitical contexts influence the design and functionality of bureaucratic organizations, and what implications does this have for their operational effectiveness in...
-
Assume you work for a car manufacturing firm , and you are trying to estimate and budget for the upcoming year . What types of factors would need to be considered ? What departments would be involved...
-
During January the following purchase transactions occurred: 8-Jan Purchased $5,900 of merchandise from The Chocolate Shop. Terms 2/15, n/45, FOB shipping point. The Candy Store prepaid...
-
A company assigns overhead cost to completed jobs on the basis of 120% of direct labor cost. The job cost sheet for Job 413 shows that $12,000 in direct materials has been used on the job and that...
-
Explain how two samples can have the same mean but different standard deviations. Draw a bar graph that shows the two samples, their means an standard deviations as error bars. T S
-
For the exercise in this topic, base your answers on the potentials listed in Table 6M.1 or Appendix 2B, with the exception of the reduction and oxidation of water at pH = 7: The anode of an...
-
(a) When the pH of 0.10 m HClO 2 (aq) was measured, it was found to be 1.2. What are the values of K a and pK a of chlorous acid? (b) The pH of a 0.10 m propylamine, C 3 H 7 NH 2 , aqueous solution...
-
Balance each of the following skeletal equations by using oxidation and reduction half-reactions. All the reactions take place in acidic solution. Identify the oxidizing agent and reducing agent in...
-
Cash Transaction Type (All) Borrow from the bank Collect from customers Iss Collect from customers Pay dividends to stockholders Pay interst on borrowing Pay salaries and wages to employees Purchase...
-
Preparation of Page One of Form 1120 Complete the header section to include company information, with no errors. Consider the following as you fill out this section of the form: Significance of the...
-
References: McLean, S. (2018). Exploring Interpersonal Communication (2nd ed) . VitalSource Bookshelf. vbk://9781453390429 Challenge : I have no idea how to argue with someone in a polite manner ...

Study smarter with the SolutionInn App


