Consider the hypothetical reaction, A 2 + B 2 2AB, where the rate law is: The
Question:
Consider the hypothetical reaction, A2 + B2 → 2AB, where the rate law is:
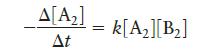
The value of the rate constant at 302°C is 2.45 × 10-4 L mol-1 s-1, and at 508°C the rate constant is 0.891 L mol-1 s-1. What is the activation energy for this reaction? What is the value of the rate constant for this reaction at 375°C?
Transcribed Image Text:
Δ[Α2] k[Ag][B2] ΔΕ
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (10 reviews)
a The activation energy for t...View the full answer

Answered By

Brian Otieno
I'm Brian , an experienced professional freelancer with countless hours of success in freelancing many subjects in different disciplines. Specifically, I have handled many subjects and excelled in many disciplines. I have worked on many Computer Science projects and have been able to achieve a lot in that field. Additionally, I have handled other disciplines like History, Humanities, Social Sciences, Political science, Health care and life science, and Religion / Theology. My experience generally in these subjects has made me able to deliver high-quality projects in a very timely fashion. I am very reliable at my job and will get the work done in time, no matter what. In Addition, I have managed to ensure that the work meets my client's expectations and does not cause an error. I am a hard-working and diligent person who is highly responsible for everything I do. Generally, Freelancing has made me more accountable for doing my job. Additionally, I have had a passion for writing for the last seven years in this field.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Consider the hypothetical reaction A + B + 2C 2D + 3E In a study of this reaction, three experiments were run at the same temperature. The rate is defined as 2d[B]/dt. Experiment 1: [A]0 = 2.0 M [B]0...
-
Consider the hypothetical reaction B E + F which is assumed to occur by the mechanism Where B* represents a B molecule with enough energy to surmount the reaction energy bar70. Consider the following...
-
Consider the hypothetical reaction A + B + 2C 2D + 3E where the rate law is An experiment is carried out where [A]0 = 1.0 Ã 10-2 M, [B]0 = 3.0 M, and [C]0 = 2.0 M. The reaction is started, and...
-
Suppose that in a particular area the consumption of water varies tremendously throughout the year, with average household summer use exceeding winter use by a great deal. What effect would this have...
-
The diameters of fully grown white oak trees are normally distributed, with a mean of 3.5 feet and a standard deviation of 0.2 foot, as shown in the figure. Random samples of size 16 are drawn from...
-
Write a method called depthSum that returns the sum of the values stored in a binary tree of integers weighted by the depth of each value. The method should return the value at the root, plus 2 times...
-
Repeat Example 7.3 using \(10 \mathrm{~kg} / \mathrm{h}\) of solvent in each stage. Data From Example 7.3:- The feed of Example 7.2 is extracted three times with pure chloroform at 298 K, using 8...
-
At December 31, Folgeys Coffee Company reports the following results for its calendar year. Cash sales . . . . . . . . . . . . $ 900,000 Credit sales . . . . . . . . . . . 300,000 Its year- end...
-
Lab-created diamonds have become an alternative to traditionally mined diamonds. There are concerns, however, that these artificial diamonds may undermine diamond values and the reputation of natural...
-
The irreversible losses in the penstock and its inlet and those after the exit of the draft tube are estimated to be 7 m. The elevation difference between the reservoir surface upstream of the dam...
-
A certain substance, initially present at 0.0800 M, decomposes by zero-order kinetics with a rate constant of 2.50 102 -2 mol L -1 s - 1 . Calculate the time (in seconds) required for the system to...
-
The reaction A(aq) + B(aq) products(aq) was studied, and the following data were obtained: What is the order of the reaction with respect to A? What is the order of the reaction with respect to B?...
-
Talk with a small business owner about the strategic planning process he or she uses. Do they have a mission statement? Marketing goals and objectives? A marketing plan? What are the major issues he...
-
Something is Brewing: 1. Random Walk: Weather is extremely good and Ali start randomly walking from U.E.T. central library (0,0). At each time step, Ali walks one meter in a random direction, either...
-
Question 1: Write a program to compute LCM in Python.
-
What has been your experience with emergency management? What are the responsibilities of emergency service providers in criminal justice? Name 2 emergency management certification programs. What are...
-
Question 1: Write a program to convert Kilometers to Miles, Question 2: Write a program to convert Celsius to Fahrenheit. (In Python)
-
Explain and demonstrate graphically, the short-run and long-run effects of an increase in the money supply using the AD-AS model.
-
Teachers' Retirement System of the City of New York offers several types of investments for its members. Among the choices are investments with fixed and variable rates of return. There are several...
-
Why did management adopt the new plan even though it provides a smaller expected number of exposures than the original plan recommended by the original linear programming model?
-
A 1.75 mole sample of an ideal gas is compressed isothermally from 62.0 L to 19.0 L using a constant external pressure of 2.80 atm. Calculate q, w, U, and H.
-
For each of the compounds below, locate the lone pair adjacent to a positive charge and draw the resonance structure: a. b. c. N.
-
Assume the following simplified dependence of the pressure in a ventricle of the human heart as a function of the volume of blood pumped. P s , the systolic pressure, is 120. mm Hg, corresponding to...
-
How does strategic delegation optimize organizational efficiency and resource allocation while mitigating decision-making bottlenecks?
-
Using scholarly resources which do you feel is the most limiting factor in generating innovations: the idea for an innovation, or the organizational processes to support innovation? Why? Do you think...
-
How can leaders employ delegation frameworks, such as the RACI (Responsible, Accountable, Consulted, and Informed) matrix, to clarify roles and responsibilities and streamline decision-making...

Study smarter with the SolutionInn App


